Invention (Patent Application Publication): Komistek
RD. Maintaining proper mechanics THA. US20120221115A1 (2012).
US20120221115A1 US
Inventor: Richard D. Komistek
Current Assignee: DePuy Ireland ULC
Worldwide applications 2011 US 2012 AU CN EP WO EP EP CN EP JP 2013 ZA 2015 US 2016 AU JP US 2018 US AU
Application US13/034,226 events:
2011-02-24 Priority to US13/034,226
2011-02-24 Application filed by Individual
2012-08-30 Publication of US20120221115A1
2015-05-05 Publication of US9023112B2
2015-05-05 Application granted
Status: Active
2031-02-24 Anticipated expiration
Maintaining proper mechanics THA
Richard D.
Komistek
Abstract
A prosthetic hip joint
comprising: (a) a femoral component including a femoral head; and, (b) an
acetabular component including an acetabular cup and an acetabular cup insert,
the acetabular cup insert sized to receive the femoral head, where the femoral
head is sized to have a spherical center that matches a spherical center of a
patient's native femoral head, where the acetabular cup is sized to have a
cavity with a spherical center that matches a spherical center of a cavity of a
patient's native acetabulum and, where the femoral head center of the femoral
component is concentric with the center of the cavity of the acetabular cup.
Description
RELATED ART
1. Field of the Invention
The present disclosure relates to orthopedic hip implants,
components thereof, and methods of preparing native tissue for implantation of
a foreign object, as well as methods of implanting foreign objects such as
orthopedic hips and components thereof.
2. Brief Discussion of Related Art
A common problem in artificial hips is dislocation resulting
from the ball of the femoral head no longer being fully seated within the
acetabular cup. Dislocation is particularly problematic immediately after
artificial hip replacement or revision surgery. As those skilled in the art are
aware, soft tissues surrounding the natural joint are damaged or removed during
surgery in order to make way for the replacement orthopedic implant. Even in
circumstances of artificial joint revision surgery, soft tissues are damaged to
gain access to the artificial joint.
Dislocation is problematic in numerous respects. First,
dislocation creates obvious kinematic problems as the joint components are not
aligned to function as designed or intended. Second, dislocation usually
results in joint pain from the unintended loads placed on surrounding tissues.
Third, dislocation usually results in swelling of tissues surrounding the
joint. Fourth, dislocation can create “popping” sounds that correlate with the
ball entering and exiting the cup repetitively. Fifth, dislocation causes
moments to be created in the joint. Sixth, dislocation leads to premature wear
of the cup and/or femoral head, thereby increasing the likelihood of joint
failure or loosening of the joint.
Many hypothesizes exist as to the cause of dislocation as
well as methods and devices to reduce or inhibit dislocation. For example,
certain orthopedic hip joints include permanent retention rings to lock the
femoral head into the acetabular cup. But these retention rings come at a
price—decreased range of motion. As the age of patients undergoing joint
replacement and revision surgeries drops and activity level of older adults
increases, decreased range of motion is not a trade-off most patients are
willing to make to inhibit dislocation.
Another problem with existing THA is the incidence of
femoral head separation within the acetabular cup, leading to the femoral head
sliding out in the superior-lateral direction and then back in the
inferior-medial direction. This incidence of sliding of the femoral head within
the acetabular cup leads to the observation that present day THA do not
function as a revolute joint, but rather have induced undesirable shear forces
that do not exist in the native hip joint. This inducement of femoral head
separation may be a main reason for the occurrence of hip dislocation.
INTRODUCTION TO THE INVENTION
It is a first aspect of the present invention to provide an
orthopedic hip joint comprising: (a) an implantable femoral component having a
first resonant frequency; (b) an implantable acetabular component having a
second resonant frequency; and, (c) a vibrational damper mounted to at least one
of the implantable femoral component and the implantable acetabular component,
where a frequency resulting from interaction between the femoral component and
the acetabular component approximates a resonant frequency of at least one of a
femur, a pelvis, and connective tissue around a hip joint.
In a more detailed embodiment of the first aspect, the
implantable femoral component includes a femoral stem, a femoral neck, and a
femoral head, the femoral neck is separable from the femoral head and, the vibrational
dampener comprises at least a portion of the femoral neck. In yet another more
detailed embodiment, the implantable femoral component includes a femoral stem,
a femoral neck, and a femoral head, the femoral neck is separable from the
femoral head and, the vibrational dampener interposes the femoral neck and the
femoral head. In a further detailed embodiment, the implantable femoral
component includes a femoral stem, a femoral neck, and a femoral head and, the
vibrational dampener comprises a sleeve wrapped around the femoral stem. In
still a further detailed embodiment, the implantable acetabular component
includes an acetabular cup and an acetabular cup insert and, the vibrational
dampener interposes the acetabular cup and the acetabular cup insert. In a more
detailed embodiment, the implantable acetabular component includes an
acetabular cup and an acetabular cup insert and, the vibrational dampener is
mounted to a bone side of the acetabular cup. In a more detailed embodiment,
the vibrational damper comprises at least one of silicone rubber, elastic
silicone rubber, gutta percha, saline rubber, gore-tex, polystyrene,
polytetrafluoroethylene, nylon, polyethylene, polyester, silk, polyethylene
teraphthalate, and polyvinyl alcohol-hydrogel.
It is a second aspect of the present invention to provide a
method of reducing propagation of vibrations through at least one component of
an orthopedic hip joint, the method comprising mounting a vibrational damper to
at least one of a femoral component and an acetabular component of an
orthopedic hip joint.
In a more detailed embodiment of the second aspect, the
femoral component includes a femoral stem, a femoral neck, and a femoral head
and, the vibrational dampener comprises a sleeve wrapped around the femoral stem.
In yet another more detailed embodiment, the femoral component includes a
femoral stem, a femoral neck, and a femoral head and, the vibrational dampener
interposes the femoral neck and femoral head. In a further detailed embodiment,
the acetabular component includes an acetabular cup and an acetabular cup
insert and, the vibrational dampener interposes the acetabular cup and the
acetabular cup insert. In still a further detailed embodiment, the acetabular
component includes an acetabular cup and an acetabular cup insert and, the
vibrational dampener is mounted to a bone side of the acetabular cup.
It is a third aspect of the present invention to provide an
orthopedic hip joint comprising: (a) an implantable femoral component includes
a first magnet exhibiting a first magnetic field; and, (b) an implantable
acetabular component includes a second magnet exhibiting a second magnetic
field.
In a more detailed embodiment of the third aspect, the
implantable femoral component includes a femoral stem, a femoral neck, and a
femoral head, the femoral head includes the first magnet and, the first magnet
is oriented so that upon implantation its positive pole is nearer the second
magnet than a negative pole of the first magnet. In yet another more detailed
embodiment, the implantable acetabular component includes an acetabular cup and
an acetabular cup insert and, the second magnet is oriented so that upon
implantation its negative pole is nearer the positive pole of the first magnet
than is a positive pole of the second magnet. In a further detailed embodiment,
the implantable acetabular component includes an acetabular cup and an
acetabular cup insert and, the second magnet is oriented so that upon
implantation its positive pole is nearer the positive pole of the first magnet
than is a negative pole of the second magnet. In still a further detailed
embodiment, the implantable femoral component includes a femoral stem, a
femoral neck, and a femoral head, the femoral head includes the first magnet
and, the first magnet is oriented so that upon implantation its negative pole
is nearer the second magnet than a positive pole of the first magnet. In a more
detailed embodiment, the implantable acetabular component includes an
acetabular cup and an acetabular cup insert and, the second magnet is oriented
so that upon implantation its negative pole is nearer the negative pole of the
first magnet than is a positive pole of the second magnet. In a more detailed
embodiment, the implantable acetabular component includes an acetabular cup and
an acetabular cup insert and, the second magnet is oriented so that upon
implantation its positive pole is nearer the negative pole of the first magnet
than is a negative pole of the second magnet. In another more detailed
embodiment, the acetabular component comprises an acetabular cup and an
acetabular cup insert and, the second magnet is part of the acetabular cup. In
yet another more detailed embodiment, the acetabular component comprises an
acetabular cup and an acetabular cup insert and, the second magnet is part of
the acetabular cup insert. In still another more detailed embodiment, the
acetabular component comprises an acetabular cup and an acetabular cup insert,
the acetabular component includes a plurality of magnets, where the plurality
of magnets include the second magnet and, at least two of the plurality of
magnets are oriented so that a negative pole of each magnet is upon
implantation closer to a femoral head of the femoral component than is a
positive pole of each magnet.
In yet another more detailed embodiment of the third aspect,
the at least two of the plurality of magnets are symmetrically oriented with
respect to an axis extending through the acetabular component. In still another
more detailed embodiment, the at least two of the plurality of magnets are
asymmetrically oriented with respect to an axis extending through the
acetabular component. In a further detailed embodiment, the acetabular
component comprises an acetabular cup and an acetabular cup insert, the
acetabular component includes a plurality of magnets, where the plurality of
magnets include the second magnet and, at least two of the plurality of magnets
are oriented so that a positive pole of each magnet is upon implantation closer
to a femoral head of the femoral component than is a negative pole of each
magnet. In still a further detailed embodiment, the at least two of the
plurality of magnets are symmetrically oriented with respect to an axis
extending through the acetabular component. In a more detailed embodiment, the
at least two of the plurality of magnets are asymmetrically oriented with
respect to an axis extending through the acetabular component.
It is a fourth aspect of the present invention to provide a
method of decreasing impact forces between orthopedic hip joint components, the
method comprising: (a) associating a first magnetic field with a femoral
component of an orthopedic joint, the first magnetic field having a positive
pole and a negative pole; and, (b) associating a second magnetic field with an
acetabular component of the orthopedic joint, the second magnetic field having
a positive pole and a negative pole, where at least one of the positive poles
and the negative poles are nearer one another than is the other of the positive
poles and the negative poles.
In a more detailed embodiment of the fourth aspect, the act
of associating the first magnetic field with the femoral component includes
including a magnet as part of a femoral head, the magnet of the femoral head is
oriented so the positive pole is nearer the acetabular component than is the
negative pole, the act of associating the second magnetic field with the
acetabular component includes including a magnet as part of at least one of an
acetabular cup and an acetabular cup insert and, the magnet of the acetabular
component is oriented so the positive pole is nearer the positive pole of the
magnet of the femoral component than is the negative pole. In yet another more
detailed embodiment, the act of associating the first magnetic field with the
femoral component includes including a magnet as part of a femoral head, the
magnet of the femoral head is oriented so the negative pole is nearer the
acetabular component than is the positive pole, the act of associating the
second magnetic field with the acetabular component includes including a magnet
as part of at least one of an acetabular cup and an acetabular cup insert and,
the magnet of the acetabular component is oriented so the negative pole is
nearer the positive pole of the magnet of the femoral component than is the
positive pole. In a further detailed embodiment, the magnet of the femoral
component is part of the acetabular cup. In still a further detailed
embodiment, the magnet of the femoral component is part of the acetabular cup
insert. In a more detailed embodiment, the step of associating a second
magnetic field with the acetabular component of the orthopedic joint includes
establishing a plurality of positive poles and a plurality of negative poles.
It is a fifth aspect of the present invention to provide a
method of retarding dislocation between a femoral component and an acetabular
component of an orthopedic hip joint, the method comprising: (a) associating a
first magnetic field with a femoral component of an orthopedic joint, the first
magnetic field having a positive pole and a negative pole; and, (b) associating
a second magnetic field with an acetabular component of the orthopedic joint,
the second magnetic field having a positive pole and a negative pole, where an
attraction force between one of the positive poles and one of the negative
poles operates to retard dislocation between the femoral component and the
acetabular component upon implantation.
In a more detailed embodiment of the fifth aspect, the act
of associating the first magnetic field with the femoral component includes
including a magnet as part of a femoral head, the magnet of the femoral head is
oriented so the positive pole is nearer the acetabular component than is the
negative pole, the act of associating the second magnetic field with the
acetabular component includes including a magnet as part of at least one of an
acetabular cup and an acetabular cup insert and, the magnet of the acetabular
component is oriented so the negative pole is nearer the positive pole of the
magnet of the femoral component than is the positive pole. In yet another more
detailed embodiment, the act of associating the first magnetic field with the
femoral component includes including a magnet as part of a femoral head, the
magnet of the femoral head is oriented so the negative pole is nearer the
acetabular component than is the positive pole, the act of associating the
second magnetic field with the acetabular component includes including a magnet
as part of at least one of an acetabular cup and an acetabular cup insert and,
the magnet of the acetabular component is oriented so the positive pole is
nearer the negative pole of the magnet of the femoral component than is the
negative pole. In a further detailed embodiment, the magnet of the femoral
component is part of the acetabular cup. In still a further detailed
embodiment, the magnet of the femoral component is part of the acetabular cup
insert. In a more detailed embodiment, the step of associating a second
magnetic field with the acetabular component of the orthopedic joint includes
establishing a plurality of positive poles and a plurality of negative poles.
It is a sixth aspect of the present invention to provide a
prosthetic hip joint comprising: (a) a femoral component including a femoral
head; and, (b) an acetabular component including an acetabular cup and an
acetabular cup insert, the acetabular cup insert sized to receive the femoral
head, where the femoral head is sized to have a spherical center that matches a
spherical center of a patient's native femoral head, where the acetabular cup
is sized to have a cavity with a spherical center that matches a spherical
center of a cavity of a patient's native acetabulum and, where the femoral head
center of the femoral component is concentric with the center of the cavity of
the acetabular cup.
In a more detailed embodiment of the sixth aspect, the
spherical center of the patient's native femoral head is determined from the
interface of the native femoral head with the native acetabulum during walking.
In yet another more detailed embodiment, the spherical center of the patient's
native acetabulum is determined from the interface of the native femoral head
with the native acetabulum during walking. In a further detailed embodiment,
the patient's native femoral head includes cartilage mounted to the native
femoral head. In still a further detailed embodiment, the cavity of the
patient's native acetabulum includes cartilage mounted to the native
acetabulum. In a more detailed embodiment, a radial thickness of the acetabular
cup is nonuniform along a circumferential length. In a more detailed
embodiment, a radial thickness of the femoral head is nonuniform along a
circumferential length. In another more detailed embodiment, an outer aspect of
the acetabular cup is nonspherical and an inner aspect of the acetabular cup is
spherical. In yet another more detailed embodiment, an outer aspect of the
acetabular cup is spherical and an inner aspect of the acetabular cup is
nonspherical.
It is a seventh aspect of the present invention to provide a
method of designing an orthopedic hip joint implant, the method comprising: (a)
conducting a kinematic analysis of a population eligible for hip replacement
surgery; (b) establishing contact points between a native femur and a native
acetabulum for each person in the population using the kinematic analysis; (c)
creating an imaginary sphere that correlates with the contact points for each
person in the population; (d) determining a dimension of the imaginary sphere
for each person in the population including at least one of radius, diameter,
circumference, and center point; and, (e) designing at least one of a femoral
component and an acetabular component using the dimension of the imaginary
sphere for each person in the population.
In a more detailed embodiment of the seventh aspect, the
determining step includes determining the center point of the imaginary sphere,
where the center point represents the anatomical spherical center and, the
designing step includes designing the femoral component to have a femoral ball
with a spherical curvature, the spherical curvature corresponding to an imaginary
prosthetic sphere having a center that is the same as the anatomical spherical
center. In yet another more detailed embodiment, the population comprises a
single person. In a further detailed embodiment, the population comprises a
plurality of persons having at least one common trait taken from the group of
age, gender, race, height, bone size. In still a further detailed embodiment,
the conducting step includes observing a hip joint of each person in the
population, where the observation takes place while the hip joint is under
weight-bearing stress. In a more detailed embodiment, the observation includes
using at least one of fluoroscopy, magnetic resonance imaging, CT imaging,
ultrasound. In a more detailed embodiment, the conducting step includes
observing a hip joint of each person in the population and, the conducting step
includes creating a three dimensional model of the hip joint for each person in
the population. In another more detailed embodiment, the establishing step
includes utilizing a collision detection analysis to establish the contact
points between the native femur and the native acetabulum for each person in
the population using the three dimensional model of the hip joint. In yet
another more detailed embodiment, the invention further includes mapping a
location of the imaginary sphere for each person in the population with respect
to boney landmarks.
It is a ninth aspect of the present invention to provide a
method of fabricating an orthopedic hip joint, the method comprising: (a)
conducting a kinematic analysis of a population eligible for hip replacement
surgery; (b) establishing contact points between a native femur and a native
acetabulum for each person in the population using the kinematic analysis; (c)
creating a sphere that correlates with the contact points for each person in
the population; (d) determining a dimension of the sphere for each person in
the population including at least one of radius, diameter, circumference, and
center point; (e) designing at least one of a femoral component and an
acetabular component using the dimension of the sphere for each person in the
population; and, (f) fabricating at least one of the femoral component and the
acetabular component.
In a more detailed embodiment of the ninth aspect, the
determining step includes determining the center point of the imaginary sphere,
where the center point represents the anatomical spherical center and, the
designing step includes designing the femoral component to have a femoral ball
with a spherical curvature, the spherical curvature corresponding to an
imaginary prosthetic sphere having a center that is the same as the anatomical
spherical center. In yet another more detailed embodiment, the population
comprises a single person. In a further detailed embodiment, the population
comprises a plurality of persons having at least one common trait taken from
the group of age, gender, race, height, bone size. In still a further detailed
embodiment, the conducting step includes observing a hip joint of each person
in the population, where the observation takes place while the hip joint is
under weight-bearing stress. In a more detailed embodiment, the observation
includes using at least one of fluoroscopy, magnetic resonance imaging, CT
imaging, ultrasound. In a more detailed embodiment, the conducting step
includes observing a hip joint of each person in the population and, the
conducting step includes creating a three dimensional model of the hip joint
for each person in the population. In another more detailed embodiment, the
establishing step includes utilizing a collision detection analysis to
establish the contact points between the native femur and the native acetabulum
for each person in the population using the three dimensional model of the hip
joint. In yet another more detailed embodiment, the invention further includes
mapping a location of the imaginary sphere for each person in the population
with respect to boney landmarks.
It is a tenth aspect of the present invention to provide an
acetabular cup comprising a bowl-shaped wall at least partially delineating a
concavity, the bowl-shaped wall including a top perimeter demarcating a first
opening through the bowl-shaped wall, the bowl-shaped wall also demarcating a
second opening sized to allow throughput of at least a portion of a femoral
head ligament.
In a more detailed embodiment of the tenth aspect, the
invention also includes at least one tab operatively coupled to the wall, the
at least one tab including a through hole. In yet another more detailed
embodiment, the invention also includes a plurality of tabs circumferentially
distributed about the top perimeter of the wall, each of the plurality of tabs
having a through hole. In a further detailed embodiment, the invention also
includes a plurality of guide pins each sized to allow insertion into the
through hole of each tab.
It is an eleventh aspect of the present invention to provide
a femoral component of a prosthetic hip joint comprising a femoral stem adapted
to be inserted into the intramedullary canal of a femur, the femoral stem
coupled to a femoral neck, the femoral neck extending proximally away from the
femoral stem, the femoral neck operatively coupled to a femoral ball mounted to
a proximal end of the femoral neck, where the femoral ball includes a proximal
cavity.
In a more detailed embodiment of the eleventh aspect, the
proximal cavity of the femoral ball is a through hole extending through the
femoral ball. In yet another more detailed embodiment, the proximal cavity
extends into the femoral neck. In a further detailed embodiment, the proximal
cavity extends into the femoral stem. In still a further detailed embodiment,
the femoral stem, femoral neck, and femoral ball comprise a single piece. In a
more detailed embodiment, the proximal cavity has at least one of a circular
cross-section, a rectangular cross-section, and an irregular cross-section.
It is a twelfth aspect of the present invention to provide a
method of mounting an acetabular component to a patient, the method comprising:
(a) positioning and aligning an acetabular jig with respect to an acetabulum,
where the acetabular jig includes a bowl-shaped wall having a through hole
accommodating throughput of a portion of a femoral head ligament attached to
the acetabulum; (b) drilling reference holes proximate the acetabulum using the
acetabular jig as a guide; and, (c) inserting a pin into each reference hole,
where the positioning and aligning step includes inserting the portion of the
femoral head ligament attached to the acetabulum through the through hole of
the acetabular jig.
In a more detailed embodiment of the twelfth aspect, the
method further includes positioning a guide cup with respect to the pelvis
using the pins, mounting a guide pin to the acetabulum while the guide cup is
in position and, removing the guide cup after the guide pin is mounted to the
acetabulum.
It is a thirteenth aspect of the present invention to
provide a prosthetic hip joint comprising: (a) a femoral component including a
femoral head with a femoral head cavity; and, (b) an acetabular component
including an acetabular cup and an acetabular cup insert, the acetabular cup
insert and the acetabular cup each having a through hole, where the through
holes overlap a location of a native femoral head ligament.
In a more detailed embodiment of the thirteenth aspect, the
femoral head cavity is sized to receive a portion of a native femur that
remains attached to the native femoral head ligament. In yet another more
detailed embodiment, the femoral head cavity extends into a neck of the femoral
component. In a further detailed embodiment, the femoral head cavity extends
through a neck of the femoral component and into a shaft of the femoral
component. In still a further detailed embodiment, the through holes of the
acetabular cup and acetabular cup insert are oriented to align with a location
where a femoral head ligament is mounted to an acetabulum.
It is a fourteenth aspect of the present invention to
provide a method of implanting an orthopedic hip joint, the method comprising:
(a) implanting and mounting an acetabular component to a native acetabulum; (b)
implanting and mounting a femoral component to a native femur; and, (c)
maintaining a connection between a native femoral head ligament and at least
one of the native acetabulum and the native femur after implanting and mounting
the acetabular component and the femoral component.
In a more detailed embodiment of the fourteenth aspect, the
method further includes reshaping a portion of a native femoral head attached
to the native femoral head ligament to create a femoral revision and, coupling
the femoral component to the femoral revision. In yet another more detailed
embodiment, the invention further includes severing the native femoral head
from the native femur, wherein the femoral revision comprises a femoral bone
insert mounted to the native femoral head ligament, and wherein the act of
coupling the femoral component to the femoral revision includes inserting the
femoral bone insert into a cavity of the femoral component. In a further
detailed embodiment, the cavity extends into a neck of the femoral component.
In still a further detailed embodiment, the cavity extends through a neck of
the femoral component and into a shaft of the femoral component.
It is a fifteenth aspect of the present invention to provide
a method of implanting at least one orthopedic hip joint component, the method
comprising: (a) implanting and mounting at least one of an acetabular component
to a native acetabulum and a femoral component to a native femur; and, (b)
maintaining a connection between a native femoral head ligament and at least
one of the native acetabulum and the native femur.
In a more detailed embodiment of the fifteenth aspect, the
implanting act includes mounting the acetabular component to the native
acetabulum and, the acetabular component includes a cup having an orifice
through which the native femoral head ligament extends. In yet another more
detailed embodiment, the implanting act includes mounting the femoral component
to the native femur and, the femoral component includes a cavity to receive at
least a portion of the native femur connected to the native femoral head
ligament. In a further detailed embodiment, the invention further includes
reshaping a portion of a native femoral head attached to the native femoral
head ligament to create a femoral revision, implanting and mounting the femoral
component to the native femur and, coupling the femoral component to the
femoral revision. In still a further detailed embodiment, the invention further
includes severing the native femoral head from the native femur, wherein the
femoral revision comprises a femoral bone insert, and wherein the act of
coupling the femoral component to the femoral revision includes inserting the
femoral bone insert into a cavity of the femoral component. In a more detailed
embodiment, the implanting act includes implanting and mounting the acetabular
component to the native acetabulum and, the implanting act includes implanting
and mounting the femoral component to the native femur. In a more detailed
embodiment, the invention further includes mounting a first portion of a tether
to at least one of the native acetabulum and the acetabular component, and
mounting a second portion of the tether to at least one of the native femur and
the femoral component.
It is a sixteenth aspect of the present invention to provide
an acetabular component of a prosthetic hip joint comprising: (a) an acetabular
cup adapted to be mounted to a native acetabulum; (b) a first acetabular cup
insert to be mounted to the acetabular cup and repositionable with respect to
the acetabular cup; and, (c) a second acetabular cup insert to be mounted to
the first acetabular cup insert and repositionable with respect to the first acetabular
cup insert, the first acetabular cup insert interposing the second acetabular
cup insert and the acetabular cup.
In a more detailed embodiment of the sixteenth aspect, the
acetabular cup includes a circumferential groove on an interior surface thereof,
the first acetabular cup includes a projection that is received within the
circumferential groove and, the projection is repositionable with respect to
the circumferential groove. In yet another more detailed embodiment, the first
acetabular cup insert includes a circumferential groove on an exterior surface
thereof, the acetabular cup includes a projection on an interior surface
thereof and, the circumferential groove is repositionable with respect to the
projection. In a further detailed embodiment, the first acetabular cup insert
includes a circumferential groove on an interior surface thereof, the second
acetabular cup includes a projection that is received within the
circumferential groove and, the projection is repositionable with respect to the
circumferential groove. In still a further detailed embodiment, the second
acetabular cup insert includes a circumferential groove on an exterior surface
thereof, the first acetabular cup insert includes a projection on an interior
surface thereof and, the circumferential groove is repositionable with respect
to the projection. In a more detailed embodiment, the first acetabular cup
insert is slidably repositionable with respect to the acetabular cup within a
first plane, the first acetabular cup insert is slidably repositionable with
respect to the second acetabular cup insert within a second plane and, the
first plane is generally perpendicular with respect to the second plane. In a
more detailed embodiment, the first acetabular cup insert is rotationally
repositionable with respect to the acetabular cup and, the first acetabular cup
insert is slidably repositionable with respect to the second acetabular cup
insert. In another more detailed embodiment, the first acetabular cup insert is
slidably repositionable with respect to the acetabular cup and, the first
acetabular cup insert is rotationally repositionable with respect to the second
acetabular cup insert.
It is a seventeenth aspect of the present invention to
provide a method of assembling a mobile bearing acetabular component of a
prosthetic hip joint, the method comprising: (a) mounting a first acetabular
cup insert to an acetabular cup, where mounting the first acetabular cup insert
to the acetabular cup includes repositioning the first acetabular cup insert
with respect to the acetabular cup without disengaging the first acetabular cup
insert from the acetabular cup; and, (b) mounting a second acetabular cup
insert to the first acetabular cup insert, where mounting the second acetabular
cup insert to the first acetabular cup includes repositioning the second
acetabular cup insert with respect to the first acetabular cup insert without
disengaging the second acetabular cup insert from the first acetabular cup
insert.
In a more detailed embodiment of the seventeenth aspect,
repositioning the first acetabular cup insert with respect to the acetabular
cup includes sliding the first acetabular cup insert against the acetabular cup
and, repositioning the second acetabular cup insert with respect to the first
acetabular cup insert includes sliding the second acetabular cup insert against
the first acetabular cup insert. In yet another more detailed embodiment,
repositioning the first acetabular cup insert with respect to the acetabular
cup includes rotating the first acetabular cup insert against the acetabular
cup and, repositioning the second acetabular cup insert with respect to the
first acetabular cup insert includes sliding the second acetabular cup insert
against the first acetabular cup insert. In a further detailed embodiment,
repositioning the first acetabular cup insert with respect to the acetabular
cup includes sliding the first acetabular cup insert against the acetabular cup
and, repositioning the second acetabular cup insert with respect to the first
acetabular cup insert includes rotating the second acetabular cup insert
against the first acetabular cup insert.
It is an eighteenth aspect of the present invention to
provide an acetabular component of a prosthetic hip joint comprising: (a) an
acetabular cup adapted to be mounted to a native acetabulum; and, (b) a first
acetabular cup insert to be mounted to the acetabular cup and repositionable
with respect to the acetabular cup, the first acetabular cup insert is
concurrently repositionable deeper into an interior of the acetabular cup and
repositionable outside of an outline of the acetabular cup.
In a more detailed embodiment of the eighteenth aspect, the
acetabular cup includes a circumferential groove on an interior surface
thereof, the first acetabular cup includes a projection that is received within
the circumferential groove and, the projection is repositionable with respect
to the circumferential groove. In yet another more detailed embodiment, the
first acetabular cup insert includes a circumferential groove on an exterior
surface thereof, the acetabular cup includes a projection on an interior
surface thereof and, the circumferential groove is repositionable with respect
to the projection.
It is a nineteenth aspect of the present invention to
provide a method of assembling a mobile bearing acetabular component of a
prosthetic hip joint, the method comprising mounting a first acetabular cup
insert to an acetabular cup, where mounting the first acetabular cup insert to
the acetabular cup includes concurrently repositioning the first acetabular cup
insert deeper into an interior of the acetabular cup and repositioning the
first acetabular cup insert outside of an outline of the acetabular cup.
In a more detailed embodiment of the nineteenth aspect,
repositioning the first acetabular cup insert with respect to the acetabular
cup includes sliding the first acetabular cup insert against the acetabular
cup.
It is a twentieth aspect of the present invention to provide
a method of revising a proximal aspect of a native femur to receive a femoral
component of a prosthetic hip joint, the method comprising: (a) removing a
native femoral head from a native femur; (b) reshaping a neck of the native
femur; and, (c) mounting a prosthetic femoral component onto the reshaped neck.
In a more detailed embodiment of the twentieth aspect, the
prosthetic femoral component comprises a femoral ball. In yet another more
detailed embodiment, the prosthetic femoral component comprises a femoral ball
and a femoral neck sleeve. In a further detailed embodiment, the femoral neck
sleeve is cylindrical. In still a further detailed embodiment, the femoral neck
sleeve is frustoconical.
It is a twenty-first aspect of the present invention to
provide an orthopedic hip joint comprising: (a) an implantable femoral
component having a first resonant frequency; and, (b) an implantable acetabular
component having a second resonant frequency, where a frequency resulting from
interaction between the femoral component and the acetabular component is
different from a resonant frequency of at least one of a femur, a pelvis, and
connective tissue around a hip joint.
In a more detailed embodiment of the twenty-first aspect,
the implantable femoral component includes a femoral stem, a femoral neck, and
a femoral head and, the femoral neck is separable from the femoral head. In yet
another more detailed embodiment, the implantable acetabular component includes
an acetabular cup and an acetabular cup insert.
It is a twenty-second aspect of the present invention to
provide a method of designing an orthopedic hip joint, the method comprising:
(a) creating an implantable femoral component having a first resonant
frequency; and, (b) creating an implantable acetabular component having a
second resonant frequency, where a frequency resulting from interaction between
the femoral component and the acetabular component is different from a resonant
frequency of at least one of a femur, a pelvis, and connective tissue around a
hip joint.
In a more detailed embodiment of the twenty-second aspect,
the femoral component includes a femoral stem, a femoral neck, and a femoral
head. In yet another more detailed embodiment, the acetabular component
includes an acetabular cup and an acetabular cup insert.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is an elevated perspective view of a first
exemplary prosthetic hip joint in accordance with the instant disclosure, shown
with a ghost image of the patient's natural anatomy.
FIG. 2 is an elevated perspective view of the first
exemplary prosthetic hip joint of FIG. 1, shown with a stem damper.
FIG. 3 is an elevated perspective view of the first
exemplary prosthetic hip joint of FIG. 1, shown with an acetabular cup
damper.
FIG. 4 is an elevated perspective view of the first
exemplary prosthetic hip joint of FIG. 1, shown with an acetabular cup
insert damper.
FIG. 5 is an elevated perspective view of a second
exemplary prosthetic hip joint in accordance with the instant disclosure, shown
with a ghost image of the patient's natural anatomy.
FIG. 6 is an elevated perspective view of an alternate
exemplary prosthetic hip joint, shown with indicia indicating like magnetic
fields to repel certain components.
FIG. 7 is an elevated perspective view of another
alternate exemplary prosthetic hip joint, shown with a ghost image of the
patient's natural anatomy.
FIG. 8 is a profile view of a human pelvis and proximal
femur showing concentric anatomical spheres.
FIG. 9 is a frontal view of a human pelvis and proximal
femur showing concentric anatomical spheres.
FIG. 10 is a frontal view of a human pelvis and
proximal femur showing non-concentric anatomical spheres.
FIG. 11 is a profile view of a human pelvis and
proximal femur showing non-concentric anatomical spheres.
FIG. 12 is a frontal view of a human pelvis and proximal
femur showing non-concentric anatomical spheres.
FIG. 13 is a frontal view of a human pelvis and
proximal femur showing non-concentric anatomical spheres.
FIG. 14 is a profile view of a human proximal femur
showing an anatomical sphere that is correctly selected.
FIG. 15 is a profile view of a human pelvis showing an
anatomical sphere that is correctly selected.
FIG. 16 is a profile view of a human pelvis and
proximal femur showing a common anatomical sphere center.
FIG. 17 is a frontal view of a human pelvis and
proximal femur showing a common anatomical sphere center.
FIG. 18 is an X-ray image of a preexisting hip implant
failing to have concentric centers.
FIG. 19 is another X-ray image of a different
preexisting hip implant failing to have concentric centers.
FIG. 20 is a diagram of the proximal femur and
acetabulum of the pelvis.
FIG. 21 is a profile view of a pelvis showing a femoral
head ligament extending from and attached to the acetabulum.
FIG. 22 is an overhead view of a landmark cup in accordance
with the instant disclosure shown prior to insertion into the acetabulum.
FIG. 23 is an overhead view of the landmark cup
of FIG. 22, shown subsequent to insertion into the acetabulum.
FIG. 24 is an overhead view of the landmark cup
of FIG. 22, shown subsequent to insertion into the acetabulum and with the
pins inserted.
FIG. 25 is a profile view of the acetabulum after the
landmark cup has been removed.
FIG. 26 is an overhead view of an exemplary guide pin
cup and guide pin used to orient a reamer reaming the acetabulum.
FIG. 27 is a profile view of a reamed acetabulum.
FIG. 28 is an overhead view of a permanent acetabular
cup mounted to the pelvis.
FIG. 29 is an overhead view of the permanent acetabular
cup of FIG. 28, with the guide pins removed.
FIG. 30 is a profile view of a natural human hip joint.
FIG. 31 is a profile view showing a femoral aspect of a
further exemplary hip joint in accordance with the instant disclosure, while
attached to the native pelvis.
FIG. 32 is a profile view showing the femoral aspect
and an acetabular component of the further exemplary hip joint in accordance
with the instant disclosure, while attached to the native pelvis.
FIG. 33 is a profile view showing the femoral aspect of
the further exemplary hip joint, prior to insertion into a femoral component.
FIG. 34 is a profile view showing the femoral
component, femoral aspect, and an acetabular component of the further exemplary
hip joint, while attached to the native pelvis and femur.
FIG. 35 is a profile view showing an even further
exemplary hip joint, while attached to the native pelvis and femur.
FIG. 36 includes an elevated perspective view of an
exemplary acetabular cup and a bottom view of an exemplary acetabular cup
insert.
FIG. 37 comprises two perspective views showing some of
the movement possible between the acetabular cup and acetabular cup insert
of FIG. 36.
FIG. 38 is a perspective view of the acetabular cup and
acetabular cup insert of FIG. 36, shown mounted to a pelvis.
FIG. 39 includes a top view of an exemplary acetabular
cup, a top view of an intermediate liner, and a bottom view of an exemplary
acetabular cup insert.
FIG. 40 are perspective views of the acetabular cup,
acetabular liner, and acetabular cup insert of FIG. 39, shown mounted to a
pelvis.
FIG. 41 includes a top view of an exemplary acetabular
cup, a top view of an intermediate liner, and a bottom view of an exemplary
acetabular cup insert.
FIG. 42 includes comparison views of a femoral head
prior to reshaping of the femoral neck.
FIG. 43 includes comparison views of a femoral head
prior to reshaping of the femoral neck in mounting thereto a cylindrical
sleeve.
FIG. 44 includes comparison views of a femoral head
prior to reshaping of the femoral neck in mounting thereto a conical sleeve.
FIG. 45 is a vertical cross-section of a present day
acetabular cup or cup insert having a uniform wall thickness.
FIG. 46 is a overhead view of an acetabulum shown with
various reference markings, A-E.
FIG. 47 is a vertical cross-section of an exemplary
acetabular cup or cup insert that has a non-uniform wall thickness and shape.
FIG. 48 are vertical cross-sections of exemplary
acetabular cups or cup inserts that have a non-uniform wall thickness and
shape.
FIG. 49 is are vertical cross-sections of present day
acetabular cups or cup inserts shown with an attached insert to shift the
spherical center of the acetabular component.
DETAILED DESCRIPTION
The exemplary embodiments of the present disclosure are
described and illustrated below to encompass orthopedic hip implants,
components thereof, and methods of preparing native tissue for implantation of
a foreign object, as well as methods of implanting foreign objects such as
orthopedic hips and components thereof. Of course, it will be apparent to those
of ordinary skill in the art that the preferred embodiments discussed below are
exemplary in nature and may be reconfigured without departing from the scope
and spirit of the present invention. However, for clarity and precision, the
exemplary embodiments as discussed below may include optional steps, methods,
and features that one of ordinary skill should recognize as not being a
requisite to fall within the scope of the present invention.
Referring to FIG. 1, a first exemplary prosthetic
hip joint 100 includes a femoral component 102 cooperating
with an acetabular component 104. In exemplary form, the femoral
component 102 includes a femoral stem 106 for
implantation into the proximal intramedullary canal 110 of
a femur 112 in order to secure the femoral component to the
femur. The femoral stem 106 may be fabricated from any feasible
material, including metals such as, without limitation, titanium, cobalt
chromium, and stainless steel. In this exemplary embodiment, the femoral
stem 106 includes a stem damper 120 to reduce
vibrations transmitted between the femoral stem and the femur 112 that
might contribute to loosening of the femoral stem within the intramedullary
canal. More specifically, the exemplary stem damper 120 is
wrapped around the femoral stem 106 so that the damper
interposes the stem and femur when implanted. In exemplary form, the damper 120 comprises
a sleeve that may be fabricated from one or more materials that are
biologically compatible and reduce vibrations transmitted between
the femoral stem 106 and the femur 112 including,
without limitation, silicone rubber, elastic silicone rubber, gutta percha,
saline rubber, gore-tex, polystyrene, polytetrafluoroethylene, nylon,
polyethylene, polyester, silk, polyethylene teraphthalate, polyvinyl
alcohol-hydrogel. But this is not the only damper used as part of the first
exemplary hip joint 100.
The stem damper 120 may also be inserted
between a metal sleeve that is fixated with cement and/or a bone in-growth
material, and the femoral stem 106. Therefore, the femoral
stem 106 is locked into the metal sleeve and the damper 120 is
inserted therebetween.
Referencing FIG. 2, the femoral component 102 (shown
without the stem damper 120) also includes a neck 130 coupled
to the femoral stem 106. In this exemplary embodiment,
the neck 130 includes a frustoconical end (not shown) that
engages a corresponding frustoconical cavity (not shown) formed within
a ball 136. In this exemplary embodiment, the ball 136 may
be fabricated from any feasible material, including metals and ceramics such
as, without limitation, titanium, cobalt chromium, stainless steel, and
alumina. In order to reduce vibrations transmitted between the neck 130 and
the ball 136, the frustoconical end includes a damper 140 that
interposes the neck and ball. The exemplary damper 140 comprises
a cap that conforms to the shape of the frustoconical end. Alternatively,
the damper 140 may be in the shape of a ring that circumscribes
the neck 130 of the femoral component 102.
It should be noted that when the damper 140 is
used, the frustoconical cavity formed within the ball 136 is
large enough to accommodate both the cap and the frustoconical end. As with the
foregoing damper, this exemplary damper 140 may be fabricated
from one or more materials that are biologically compatible and reduce
vibrations transmitted between the neck 130 and the ball 136 including,
without limitation, silicone rubber, elastic silicone rubber, gutta percha,
saline rubber, gore-tex, polystyrene, polytetrafluoroethylene, nylon,
polyethylene, polyester, silk, polyethylene teraphthalate, polyvinyl
alcohol-hydrogel. In addition to the dampers 120, 140 associated
with the femoral component 102, the acetabular component 104 may
also include its own dampers.
Referring to FIG. 3, the acetabular
component 104 includes an acetabular cup 150 and
an acetabular insert 152. The interior of the acetabular
cup 150 includes a semispherical cavity that receives a semispherical
aspect of the acetabular insert 152. The acetabular cup 150 includes
a damper 160 that interposes the cup and a
patient's pelvis 170. In this exemplary embodiment, the acetabular
cup 150 may be fabricated from any feasible material, including
metals such as, without limitation, titanium, cobalt chromium, and stainless
steel. In exemplary form, the damper 160 is semicircular and is
mounted to the rear of the acetabular cup 150. The overall area of
this damper 150 can be very small, less than 1.0 mm2 or can
cover the full surface area of the acetabular cup. One or more
dampers 150 may be used. By interposing the acetabular cup 150 and
the pelvis 170, vibrations transmitted between the cup and pelvis are
reduced. It is believed that vibrations transmitted between the cup 150 and pelvis 170 contribute
to loosening of the cup and joint failure. The exemplary damper 160 may
be fabricated from one or more materials that are biologically compatible and
reduce vibrations transmitted between the cup 150 and
the pelvis 170 including, without limitation, silicone rubber,
elastic silicone rubber, gutta percha, saline rubber, gore-tex, polystyrene,
polytetrafluoroethylene, nylon, polyethylene, polyester, silk, polyethylene
teraphthalate, polyvinyl alcohol-hydrogel.
Referencing FIG. 4, in order to reduce vibrations
transmitted between the acetabular component 104 and adjacent
structures, a damper 180 interposes the acetabular
insert 152 and the acetabular cup 150 (shown without the
damper 160). In this exemplary embodiment, the acetabular
insert 152 may be fabricated from any feasible material, including
metals and ceramics such as, without limitation, titanium, cobalt chromium,
stainless steel, and alumina. In exemplary form, the damper 180 is
semicircular and is mounted to the backside of the acetabular insert 152.
The overall area of this damper 180 may be very small, for
example less than 1.0 mm2, or can cover the full surface area of the acetabular
cup. Either one or more dampers can be used. By interposing the acetabular
cup 150 and the acetabular insert 152, vibrations
transmitted between the cup and insert are reduced. It is believed that
vibrations transmitted between the cup 150 and insert 152 contribute
to loosening of the cup and joint failure. The exemplary damper 180 may
be fabricated from one or more materials that are biologically compatible and
reduce vibrations transmitted between the cup 150 and the insert 152 including,
without limitation, silicone rubber, elastic silicone rubber, gutta percha,
saline rubber, gore-tex, polystyrene, polytetrafluoroethylene, nylon,
polyethylene, polyester, silk, polyethylene teraphthalate, polyvinyl
alcohol-hydrogel.
The exemplary vibrational dampers 120, 140, 160, 180 may
be utilized when the resonant frequencies of adjacent components are not the
same. Due to the presence of hip separation and sliding of the femoral head
within the acetabular cup, impulse loads and vibrational energy are transmitted
and propagated throughout the hip joint.
The natural or resonant frequency of an object is the
frequency at which that object will vibrate freely. If a varying force with a
frequency equal to the natural frequency is applied to an object, the
vibrations can become violent, a phenomenon known as resonance. Resonance is
the buildup of large vibration amplitude that occurs when a structure or an
object is excited at its natural frequency. Resonance can be either desirable
or undesirable. In the context of acoustic resonance, a desirable resonance is
exhibited by musical instruments. Conversely, undesirable resonance can lead to
mechanical failures resulting in bridges collapsing and fracturing of aircraft
wings.
The quality of the vibration and propagation of the
vibration produced by a vibrating object is dependent upon the natural
frequencies of the vibrational waves produced by the object. Some objects tend
to vibrate at a single frequency, while other objects vibrate and produce more
complex waves with a set of frequencies. If converted to a sound, these objects
create sounds that could be described as noise. The actual frequency at which
an object will vibrate at is determined by the following relationship:
frequency=speed/wavelength. The inventor has found it beneficial to determine
the natural frequency of THA implantable components and secondary structures
(e.g., bone cement) to mitigate undesirable resonance.
A first exemplary method for determining resonant frequency
of a component or tissue includes excitation of the component or tissue using,
for example a speaker, amplifying different frequencies. For example, an
accelerometer may be used on bones, attaching one tri-axial accelerometer
rigidly to the bone and then when excited, the acceleration signal, once
filtered, is used to determine the natural frequency of the bone.
Also, a Fourier Series may be used to determine the
frequency of an object in question. The Fourier Series reveals how a
mathematical series of sine and cosine terms can be used to analyze a waveform.
Once the Fourier Series is written for a waveform then the components of the
series completely describe the frequency content of the waveform. There are
four conditions that must be met in order for the Fourier Series to be useful.
1. The waveform must be periodic. This waveform must repeat
time for a Fourier Series to exist.
2. If the function has discontinuities, their number must be
finite in any period.
3. The function must contain a finite number of maxima and
minima during any period.
Those skilled in the art may be familiar with numerous other
methods that may be used to determine resonant frequencies for bodily tissue,
implantable components, and secondary structures.
When a patient experiences hip separation, once the femoral
head slides back into the acetabular cup, an impulse load is been generated,
which results in vibration being propagated throughout the hip joint. If these
vibrations are at or near resonant frequencies of bone, the implanted
components, and/or secondary structures, detrimental resonance can lead to
premature failure. To reduce this premature failure and wear, vibrational
dampers are positioned to absorb vibrations between adjacent components.
In order to determine whether vibrational dampers 120, 140, 160, 180 may
be preferred, the exemplary orthopedic hip joint components may be tested to
determine their respective resonant frequencies. When two or more hip joint
components have the same or similar resonant frequencies, one or
more vibrational dampers 120, 140, 160, 180 may
be utilized. It is to be understood that testing of orthopedic components is
not required as a prerequisite for including vibrational dampers 120, 140, 160, 180 as
part of an orthopedic hip joint.
Referring to FIG. 5, an exemplary hip implant
assembly 200 includes a femoral component 202,
an acetabular cup 204, and an acetabular insert 206. Due to
the presence of high bearing surface forces in total hip arthroplasty,
the femoral component 202 may be totally or partially fabricated
using highly magnetic materials that work in conjunction with highly magnetic
materials that may be used to fabricate the acetabular cup 204 and/or acetabular
insert 206 to reduce lower hip joint forces and/or hip separation.
In this exemplary embodiment, the femoral
component 202 includes a femoral stem 208 that is
adapted to be implanted into the femoral intramedullary canal after the femoral
bone has been properly resected. Extending proximally from the femoral
stem 208 is an integral neck 210 that includes a
threaded or conical end (not shown) adapted to receive a femoral
ball 214. The femoral ball 214 is fabricated to include a
biologically compatible metallic coating (e.g., stainless steel, titanium,
titanium alloy), which surrounds a neodymium magnetic core or other ferrous
core. Alternatively, the femoral ball 214 may be fabricated to
include one or more permanent magnets (e.g., neodymium magnet) embedded within
a biologically compatible metal substrate (e.g., stainless steel, titanium,
titanium alloy). In either circumstance, the magnetic field generated by
the femoral ball 214 represents a magnetic North Pole, which is
pulled toward any magnetic South Pole.
In order to retard dislocation of the femoral
ball 214 from the acetabular insert 206,
the acetabular cup 204 includes a biologically compatible
metallic coating 220 (e.g., stainless steel, titanium, titanium
alloy), which surrounds a ferrous core. Alternatively, the acetabular
cup 204 may be fabricated to include one or more magnets embedded
within a biologically compatible metal substrate (e.g., stainless steel,
titanium, titanium alloy). In either circumstance, the magnetic field generated
by the acetabular cup 204 represents a magnetic South Pole.
Because the force between the North Pole and the South Pole is inversely
proportional to the square of the distance between the magnetized surfaces, it
is important to reduce the distances between the Poles.
In order to reduce the distances between the poles,
the acetabular insert 206 may be fabricated using two
considerations. First, the acetabular insert 206 may be
fabricated to have a minimal thickness, thereby reducing the distance between
the femoral ball 214 and the acetabular cup 204.
Alternatively, or in addition, the acetabular insert 206 may
itself house one or more magnets oriented so that the North Pole faces toward
the acetabular cup 204 and the South Pole faces toward
the femoral ball 214. In exemplary form, an acetabular
insert 206 includes a bowl-shaped neodymium magnetic core or other
ferrous magnetic core. This core is then overmolded or encapsulated in a
biologically compatible polymer or ceramic to form a capsule 226 comprising
the bearing surface of the cup 204. In exemplary form, the mean
thickness of the capsule 226 is between 0.1 mm to 20 mm.
Referring to FIG. 6, an alternate exemplary hip
implant assembly 240 includes a femoral component 242,
an acetabular cup 244, and an acetabular insert 246.
The femoral component 242 includes a femoral stem 248 having
an integral neck 250 that includes a threaded or conical end
(not shown) adapted to receive a femoral ball 254. The femoral
ball 254 is fabricated to include a biologically compatible metallic
coating (e.g., stainless steel, titanium, titanium alloy), which surrounds a
neodymium magnetic core or other ferrous core. Alternatively, the femoral
ball 254 may be fabricated to include one or more permanent magnets
(e.g., neodymium magnet) embedded within a biologically compatible metal
substrate (e.g., stainless steel, titanium, titanium alloy). In either
circumstance, the magnetic field generated by the femoral ball 254 represents
a magnetic North Pole.
In order to decrease impact forces between the femoral
component 242 and the acetabular components 244, 246,
the magnetic field of the femoral component and the acetabular components may
be the same. Specifically, at least one of the acetabular insert 246 and
the acetabular cup 244 includes a biologically compatible
metallic coating 260 (e.g., stainless steel, titanium, titanium
alloy), which surrounds a ferrous core. Alternatively, the acetabular
cup 244 and acetabular insert 246 may be fabricated to
include one or more magnets embedded within a biologically compatible metal
substrate (e.g., stainless steel, titanium, titanium alloy). In either
circumstance, the magnetic field generated by the acetabular cup 244 and acetabular
insert 246 represents a magnetic North Pole. Because the North Poles
of the femoral component 242 and the acetabular
components 244, 246 operate to repel one another, the impact
forces between the femoral component and the acetabular components may be
reduced.
Turning to FIG. 7, another alternate exemplary hip
implant assembly 270 includes a femoral component 272,
an acetabular cup 274, and an acetabular insert 276. The
femoral component 272 includes a femoral stem 278 having
an integral neck 280 that includes a threaded or conical end
(not shown) adapted to receive a femoral ball 284. In this
embodiment, the femoral ball 284 and the most proximal
aspect 286 of the acetabular cup 274 (farthest from the
femoral shaft) both have a positive polarity (i.e., North Pole), but
a distal medial 288 and distal lateral aspect 290 of
the acetabular cup 274 have a negative polarity (i.e., South
Pole). In exemplary form, the positive-positive polarity interaction operates
to decrease the compressive forces during weight-bearing activity, while the
positive-negative polarity interaction resists dislocation and femoral ball
separation.
Referencing FIGS. 8 and 9, an additional
exemplary orthopedic hip joint 300 comprises a cup
component 302 and a femoral component 304 having
concentric spheres. More specifically, the patient's anatomy is prepared to
ensure both the cup component 302 and a femoral
component 304 have a common spherical center with respect to the
acetabulum.
Referring to FIGS. 10-13, although it has been stated
in numerous publications that the human hip is a revolute joint, whereas only
three sequential rotations are present, the actual shapes of the acetabulum and
femoral head of the femur are not pure spheres. In fact, the position of
numerous exterior points on the acetabulum and the femoral head can be recorded
and computer algorithms applied to these points to create spheres whose surface
best correlates with the recorded points. In a study conducted by the inventor,
it was determined that numerous spheres can be derived using sets of points on
the surface of the acetabulum and the femoral head. Therefore, in a
conservative sense, using sets of points on the acetabulum and on the femoral
head, one could easily derive at least fifty spheres for each of the acetabulum
and the femoral head that at least partially correlated with the recorded
points (i.e., at least some of the recorded points would comprise an exterior
point on a sphere). Therefore, if fifty spheres for the acetabulum and fifty
spheres for the femoral head were chosen, this would result in twenty-five
hundred combinations of spheres. But the inventor has determined that the
correct combination of spheres is a concentric combination derived from the
bearing surface interfaces of the acetabulum and femoral head during walking,
and is derived from the cartilage surface within the acetabulum and the
cartilage surface on the femoral head.
Referring back to FIGS. 8 and 9, in order to design
the cup component 302 and the femoral component 304, a
kinematic analysis is conducted for the intended recipient of
the orthopedic hip joint 300. This kinematic analysis defines points
on recipient's natural femoral head in contact with the acetabulum and
corresponding points on the acetabulum in contact with the femoral head.
An exemplary kinematic analysis is performed to determine
these spheres while the joint is under dynamic, weight-bearing, in vivo
conditions. During normal gait motion, these spheres maintain concentricity.
Therefore, with present imaging technology, fluoroscopy is an exemplary method
of use. But other imaging modalities, like ultrasound could be used to perform
the kinematic analysis. Under fluoroscopic surveillance, the patient performs
normal walking. Then, the patient undergoes a second clinical imaging test
using CT, MRI, or ultrasound (other technologies could also be used). In the
context of a CT scan, the scanned slices of the joint are used to create a
three dimensional (3D) model of the patient's pelvis and femoral bones. Then,
these 3D bones are overlaid onto the two dimensional fluoroscopic images. Once
all of the fluoroscopic images, or a selected chosen few fluoroscopic images
are converted to 3D, the patient's hip motion may be viewed in any chosen
plane. Using a collision detection analysis, the point of the femoral head in
contact with the pelvis and the points of the pelvis in contact with the
femoral head are determined and mapped sequentially.
If one does not have the capability to determine the correct
points on the acetabulum and the femoral head using the above mentioned
kinematic analysis, one may alternatively use trial and error to derive the
location of the concentric spheres by placing different sizes of spheres in
different locations relative to the acetabulum and the femoral head for each
patient until the spheres are concentric in multiple planes. Once the anatomical
concentricity is established for that particular patient, the location can be
mapped and relocated during surgery to ensure that the spherical centers of the
implanted components are matched to the anatomical spherical centers.
Referring to FIGS. 14-17, spheres are superimposed onto
the points that best conform to the mapped points on the pelvis and femoral
head to create individual spheres. It is important to note that these spheres
may be derived using the boney anatomy or on the cartilage. The correct sphere
for each patient may be dependent on the quality of cartilage and/or the
concentricity of the two spheres.
After the spheres are defined, the location of the center of
these spheres is defined and used a target origin (or center) for the spheres
of the implanted femoral head and the acetabular cup The center of two derived
spheres can be defined quite easily using numerous software packages and/or
using a mathematical approach. It is important to then map out the location of
the patient's anatomical sphere centers with respect to boney landmarks. The
center of the chosen pelvis sphere needs to be tracked with respect to boney
landmarks on the pelvis and the center of the femoral head sphere needs to be
tracked with respect to boney landmarks on the femur bone.
Preparation of the bones to receive prosthetic components
should be done with respect to maintaining the patient's anatomical spheres.
Therefore, after the bones have been prepared for the implanted components, the
implanted components are implanted to maintain these spherical centers.
Alternatively, a surgical navigation system or an imaging modality may be used
to locate the patient's spherical center(s) and ensure that the implanted
components are implanted to maintain the spherical center(s).
Unlike the foregoing exemplary embodiment that is
patient-specific, cost considerations may require a finite set of implant
components that differ in size from one another. This finite set of implant
components may include gender and ethnicity considerations, depending upon the
population utilized to model the implant components. By doing so, it is
anticipated that there will be more than three acetabulum spheres (presently,
patients normally received a femoral head having either a 28 or 32 or 36 mm sizing)
needed to fit everyone requiring a TKA. Then, knowing the proper acetabulum
sphere sizes, the center of these spheres is defined and used to develop proper
sizing for the acetabular cup, cup insert, and femoral ball/head components.
Proper sizes for the acetabular cup, cup insert, and femoral ball/head are
designed to maintain spherical concentricity throughout normal gait.
Maintaining proper spherical centers also leads to the
femoral stem being implanted properly so that the center of femoral head sphere
is located at the origin of the acetabular cup sphere. The centers for both of
these spheres (head and cup) are thus coincident with the anatomical center of
the acetabulum sphere taking into account the cartilage surface.
It is understood that in most sizing analysis of implants,
if a bell curve is used, there is a set number of sizes that will include 90%
of the subjects requiring that type of implant. Unfortunately, in a total hip
arthroplasty (THA), unlike other prosthesis, such as a total knee arthroplasty
(TKA), all patients receive one of three sizes. Therefore, in a perfect world,
the best outcome would be that 30% of the patients receive a THA implant that
may maintain concentric spheres. Unfortunately, this is not the case because a
slight misalignment of the implanted components will lead to the pelvis and
femoral head spheres not being concentric. Therefore, it is important to
understand and derive proper spheres that allow at least 90% of the population
to receive a THA with the ability to maintain their anatomic sphere
concentricity. Using an exemplary kinematic analysis as discussed previously
herein, one determines spherical sizes for the pelvis and spherical sizes for
the femoral head that fit a predetermined percentage of patients. Although
present day sizes are only 28, 32 and 36 mm femoral heads that are then mated
with the acetabular cup liner, this analysis may reveal that 10 to 12 sizes of
femoral heads and acetabular cup liners should be produced so that 90% of the
subjects under the bell curve could receive proper femoral head and acetabular
cup sizes that maintain their spherical concentricity. These sizes may not be
whole numbers, but rather decimal numbers. Again, it is important that each
patient receive a femoral head and acetabular component that maintains
spherical concentricity after THA implantation. Using the wrong femoral head
and/or acetabular cup insert size leads to these implanted spheres not being
concentric with the patient's anatomical spherical concentricity. This improper
sizing may lead to the inducement of shear forces, further leading to femoral
head separation and/or dislocation.
The shape of present day femoral stems is not able to
accommodate spherical concentricity due to limited options. Therefore, it is understood
that multiple neck lengths and neck angles with respect to the femoral stem may
be available to the surgeon. Therefore, once the anatomical spherical center is
found, it may be relocated using the spherical centers of the implanted
components by utilizing various stem neck options. This may be of particular
concern in case where a surgeon removes too much or not enough bone and/or the
femoral cut and/or the stem is fixated into the femoral bone at an offset
angle.
Referring to FIGS. 18 and 19, femoral head separation
in present-day THA is induced by the acetabular cup and the femoral head being
implanted in a position and/or orientation that does not coincide with the
proper spherical center of patient. Thus, this misplacement of these components
induce shear forces so that the patient's muscular structure attempts to
realign the prosthetic components to the patient's proper anatomical spherical
center. FIGS. 18 and 19 show examples of present-day implants that
were implanted and how these implants have not maintained the patient's proper
anatomical spherical center. The dotted circle represents the implanted femoral
head sphere for this patient. The half dot represents the center of this
implanted femoral head sphere. The solid circle represents the anatomical
acetabulum sphere derived from the weight-bearing contact points, on the
cartilage, for this subject during normal walking. The solid dot represents the
center of this anatomical based acetabulum sphere. Unfortunately, after
implantation, the femoral head is no longer rotating around this patient's
anatomical spherical center. But the patient's muscular structure around the
hip joint is wed to this anatomical spherical center and attempts to rotate the
hip implant around this patient's anatomical spherical center. Because the
patient's anatomical sphere center and prosthetic sphere centers (for ball and
socket) are not coincident, shear forces are created in the implanted hip joint
that lead to hip separation and/or hip dislocation.
As shown in FIGS. 20 and 21, the patient's native
anatomy includes an acetabulum 510 in the pelvis 512 that
is adapted to receive femoral head 514 at the proximal end of
the femur 516, so the femoral head is received within a cavity
defined by the acetabulum to form a ball and socket joint. In a patient's
native hip joint, the acetabulum 510 defines a cavity having a
spherical center that is concentric with the spherical center of
the femoral head 514. As the femoral head 514 pivots
with respect to the acetabulum 510, this common spherical center
orientation is maintained. But preexisting orthopedic hip joints do not
maintain this common spherical center orientation between the cavity of the
acetabulum and the femoral head.
An exemplary approach for determining and maintaining this
common spherical center orientation uses human anatomical landmarks, such as
the femoral head ligament 520. The femoral head ligament 520 is
a major constraint that is currently removed without any attempt by the surgeon
to utilize its location to define cup orientation. In contrast, this exemplary
technique includes retention of certain features of the acetabulum before an
instrument may be used to define the location of the femoral head ligament.
Referring to FIG. 22, a landmark cup or jig 530 includes
an orifice 532 sized accommodate a portion of the femoral
head ligament 520 that remains attached to the acetabulum 510.
In this exemplary embodiment, the landmark cup 530 is
bowl-shaped and includes a plurality of tabs 534. Each tab 534 includes
a through hole 536 that corresponds to the location of a
fastener used to secure an acetabular cup to the pelvis 512.
Referencing FIG. 23, the landmark cup 530 is
positioned over the acetabulum 510 so that
the orifice 532 overlies the location of the femoral head
ligament 520. The orifice 532 may take on various sizes and
various positions within the cup 530 to mark the location of the
femoral head ligament and may be used on multiple patients having variable
femoral head ligament locations in the acetabulum. More specifically, after
the orifice 532 is positioned to overlie the femoral head
ligament 520, the cup 530 is pushed against
the acetabulum 510, with the femoral head ligament extending through
the orifice. Thereafter, holes are drilled into the pelvis 512 using
the tab holes 536 as guides.
It should be noted, however, that while the landmark
cup 530 is generally in the shape of an acetabular cup, this shape is
not critical. The cup 530 may be any shape, such as circular,
elliptical, square, rectangular, etc., and could be of any size. What is
critical is retention of at least one of the anatomical reference points
associated with the acetabulum 510 so that mounting locations
and/or acetabular cup orientation can be established prior to reaming of the acetabulum.
Referring to FIG. 24, after the holes in
the pelvis 512 are drilled, pins 560 are inserted into
the holes. The landmark cup 530 is also removed, which allows
for this same orientation to be utilized later in the surgery for reaming
and permanent acetabular cup 570 positioning (see FIG. 29).
Referencing FIG. 25, the pins 560 remain
in the pelvis 512 after the landmark 530 is
removed. Thereafter, as shown in FIG. 26, the acetabulum 510 is
prepared to receive the permanent acetabular cup 570 (see FIG.
29).
Referring to FIGS. 26 and 27, it is currently difficult
for the surgeon to properly ream out the acetabulum so the acetabular cup and
cup insert are positioned correctly. To help the surgeon properly ream out the
acetabulum, after the landmark cup 530 has been removed,
a guide pin cup 580 is secured to the acetabulum using
the pins 560 that extend through corresponding openings in the
guide pin cup. The guide pin cup 580 includes an opening
exposing a portion of the acetabulum where the femoral head ligament is
located. After the guide pin cup 580 is in position,
a guide pin 582 is secured to the acetabulum at the center of
the socket, referenced with respect to the femoral head ligament, and/or other
bone or soft-tissue landmarks. This guide pin 582 may be 1.0 cm
to 20 cm in length and have diameter from 0.1 cm to 3.0 cm, for example.
The guide pin 582 may be fabricated from numerous materials such
as, without limitation, cobalt chrome, steel, titanium, tantalum, and ceramics.
Thereafter, the guide pin cup 580 is removed from over top of
the guide pin 582 and the ancillary pins 560, which
leaves the guide pin 582 mounted to the acetabulum. Using
the guide pin 582 and the ancillary pins 560 on
the pelvic bone, a reamer (not shown) is inserted in the socket and the
acetabulum is reamed uniformly and in the correct direction to create a
revised acetabulum 510′ (see FIG. 27). After reaming, the reamer
and guide pin 560 are both removed, while the ancillary
pins 560 are retained to guide the implanted cup into the socket.
Referring to FIG. 28, the permanent acetabular
cup 570 is inserted into the revised acetabulum 510′ using
the guide pins 560 so as to maintain the proper orientation necessary
to produce concentricity with the femoral head.
Referring to FIG. 29, after the acetabular
cup 570 is securely in place within the revised acetabulum 510′,
the guide pins 560 are removed and the corresponding holes filled.
Referring back to FIG. 20, during weight-bearing gait,
the femoral head ligament 520 remains taught so that the
ligament distance throughout the weight-bearing portion of the gait cycle
remains constant. The location of the femoral head ligament 520 attachment
site in the acetabulum 510 is identified as point A. The
location of the femoral head ligament attachment 520 site on
the femoral head 514 is identified as point B. Therefore, if the
line constructed from point A to point B is constant throughout the
weight-bearing portion of the gait cycle, then one may use this line to define
the location of the proper acetabulum sphere. Since points B and C are on the
same bone, a fixed body, then the distance from points B to C is always constant.
Therefore, by knowing the distances from points A to B and B to C, one may
construct a line from point A to point C, which is also a constant. Although
this analysis is planar in nature, a fourth out-of-plane point may be used to
align the longitudinal direction of the cup. By identifying the location of
the femoral head ligament 520 in the acetabulum 510,
prior to the acetabulum being prepared during surgery, the distance from
the femoral head ligament 520 to the spherical center of the
acetabular cup 570 (see FIG. 29) and the femoral head 514 is
measured to ensure that the proper spherical center has been maintained.
The femoral head ligament 520 is the only landmark within
the acetabulum 510 that can be used to define the location of
the proper spherical center. Knowing the distance from the femoral head
ligament 520 attachment site, within the acetabulum 510 to
the proper spherical center is crucial to the surgical alignment and
implantation of the acetabular cup 570 and femoral
head 514.
Although the example just described may be used to define
concentric spheres during surgery, one could use a number of methodologies to
located and/or maintain concentric spheres post THA. In an exemplary simplistic
methodology, one could attempt to define and maintain concentricity using
static x-rays, but this method would be in two-dimensions and may not properly
define concentricity in three-dimensions. This method may only allow one to
define similar circular centers. One could also use pre-operative planning
and/or imaging, such as MRI, CT scans, ultrasound and/or any other imaging
modality. Most of the imaging modalities that can presently be used are static
and may subject the patient to radiation exposure. One could also use
intra-operative surgical navigation and/or imaging modalities to locate and/or
maintain concentric spheres. Most importantly, it is important to ensure that
proper cup and femoral stem orientation is chosen to ensure concentric spheres
post THA surgery.
Referring to FIG. 30, all present-day THA surgeries
require removal of the femoral head ligament 602. But this exemplary
embodiment of a prosthetic hip joint retains the femoral head
ligament 602 if it is healthy. As stated previously, the femoral
head ligament 602 is a stabilizing mechanism in the hip joint that
couples the pelvis 603 to the femur 605.
If the femoral head ligament 602 is not
healthy, an artificial structure may be used to reinforce the femoral head
ligament. This artificial structure may be comprised of any number of materials
such as, without limitation, twine, silicone rubber, elastic silicone rubber,
gutta percha, saline rubber, gore-tex, polystyrene, polytetrafluoroethylene,
nylon, polyethylene, polyester, silk, polyethylene teraphthalate, and polyvinyl
alcohol-hydrogel. This material may be wrapped around the femoral head
ligament 602, attached to the base of the femoral head ligament attachment
site in the acetabulum, inter twined within the femoral head ligament, or used
in another manner to reinforce the strength of the femoral head ligament.
Referencing FIGS. 30 and 31, initially,
the femoral head 604 is severed from the remainder of
the femur 605. A cutting instrument (not shown) is then used to shape
a bone segment 610 from the femoral head 604, where
the bone segment remains attached to the femoral head ligament 602.
In exemplary form, the bone segment 610 is cut into a
cylindrical shape having the same length as the native femoral head 604,
with one end of the cylinder being mounted to the femoral head ligament 602.
It should be noted that the cylindrical shape is not critical and other shapes
and sizes such as, without limitation, rectangular, triangular, and rounded may
be utilized as part retaining the bone segment.
Referring to FIGS. 32-34, the exemplary prosthetic
hip joint 600 comprises an acetabular cup 620 and
an acetabular cup liner 622 each having a through orifice (not
shown) that is sized to allow throughput of the femoral head
ligament 602 and the bone segment 610. The exemplary
prosthetic hip joint 600 also comprises a femoral ball 628,
a femoral neck 630, and a femoral stem 632, where the
femoral neck is integrally formed with the femoral stem. The femoral
ball 628 includes a through hole (not shown) that is sized to allow
insertion of the bone segment 610 so the portion of the bone
segment to which the femoral head ligament is mounted is substantially flush
with the exterior arcuate surface of the ball. The neck 630 includes
a cavity 636 axially aligned with the through hole in order to
receive a portion of the bone segment 610. In this exemplary
embodiment, the cavity 636 is cylindrical.
Referring to FIGS. 32-34, implantation of the
exemplary prosthetic hip joint 600 includes utilizing a cutting
instrument to create a bone segment 610 that is mounted at one
end to the femoral head ligament 602 and free at an opposing
end. The femoral head ligament 602 during this process remains
attached to the pelvis 603. After the bone segment 610 is
cut, the bone segment and a portion of the femoral head ligament 602 are
thread through the through orifice of the acetabular cup 620.
The acetabular cup 620 is then mounted to the pelvis 603.
The bone segment 610 and a portion of the femoral head
ligament 602 are next thread through the through orifice of
the acetabular cup liner 622. The acetabular cup liner 622 is
then mounted to the acetabular cup 620. Then, the bone
segment 610 is thread through the through hole of the femoral
ball 628 so that the end of the bone segment mounted to
the femoral head ligament 602 is substantially flush with the
bearing surface of the femoral ball. And at least a portion of the
remaining bone segment 610 not received within the femoral
ball 628 is received within the femoral neck cavity 636.
The bone segment 610 may be attached to
the femoral neck 630 using numerous methodologies and
techniques. An exemplary method for use with the instant exemplary
embodiment 600 includes applying bone cement in between the bone
segment 610 and the wall(s) of the femoral neck 630 that
delineate the cavity 636. Another exemplary method includes
interposing bone ingrowth material between the bone segment 610 and
the wall(s) of the femoral neck 630 that delineate
the cavity 636.
The femoral ball 628 may alternatively be
tapered to create a cap-like indentation at the site where femoral head bone is
received. The location on the femoral ball 628, where the femoral
head bone is received thus does not have to be tapered and does not have be a
cylindrical hole. In other words, the cavity on the femoral ball 628 to
receive the modified femoral head bone may be any of a number of shapes.
The amount of bone retained from the femoral head bone may
be of any size and shape. The length of this retained femoral head bone may be
long enough to be fixated within only the femoral ball 628, or it can
be longer to insert through the femoral ball and into the femoral
neck 630 of the femoral prosthesis. Alternatively, the retained
femoral head bone may be long enough so that the distal end of the bone can
pass through the femoral ball 628, through the femoral
neck 630, through a portion of the femoral stem and into or through
the femoral shaft 632. This technique may allow the blood supply to
be maintained within the retained femoral head bone and the femoral head
ligament, thus allowing the retained femoral head bone to grow into the femur.
Referring to FIG. 35, an alternate exemplary
embodiment 700 is the same as the foregoing prosthetic hip
joint 600, except for the addition of sutures or other retention
lines 702 extending between at least two of the acetabular cup,
acetabular cup liner, the femur, the femoral neck and the pelvis. These sutures
or retention lines 702 may be utilized like suspenders, wrapping
around the acetabular cup, between the cup and the bone and then either
attaching to the femoral implant component or to the femoral bone. The
acetabular cup may include grooves 704 to allow the sutures
or other retention lines 702 to fit between the cup and the bone
and then be securely cemented to the implant and the bone or allow for the bone
to grow into the cup and/or the artificial structure.
Referencing FIGS. 36-38, an exemplary mobile
bearing acetabular component 800 comprises an acetabular
cup 802 and a repositionable cup insert 804.
The repositionable cup insert 804 includes a semicircular
rib 806 having a dove tail cross-section that extends
circumferentially on the cup insert's exterior surface. This rib 806 is
adapted to be at least partially received within a
corresponding semicircular groove 808 formed on the interior of
the cup 802. In this exemplary embodiment, the groove 808 takes
on a dove tail shape. It should be noted, however, that other rib 806 and
groove 808 shapes may be utilized such as, without limitation,
the rib 806 having a T-shape and the groove 808 having
a corresponding cavity to receive and retain the rib. Moreover, it is within
the scope of the disclosure for the rib 806 to be located on the
interior of the acetabular cup 802, while the groove 808 is
located circumferentially on the cup insert 804.
In this exemplary embodiment, during weight-bearing
activities, the cup insert 804 is locked and cannot slide and/or
extend and remains in a fixed orientation with respect to the cup 802.
During non weight-bearing activities, especially those that contribute to
dislocation, the cup insert 804 is allowed to translate along
one axis. Specifically, the rib 806 is repositionable within
the groove 808, thereby allowing the cup insert 804 to
translate along one axis with respect to the cup 802. Therefore,
pre-operatively, if preferred, one may determine the correct cup 802 orientation
so that the cup insert 804 will perfectly translate along an
axis that the patient normally uses to perform the activities that cause
femoral head dislocation. The cup insert 804 slides and/or
extends in both directions along that chosen axis. Thus, when the patient
performs non-weight bearing tasks, the cup insert 804 extends
outside of the acetabular cup 802, ensuring that the femoral head
does not dislocate. This cup insert 804 may have full freedom to
translate and/or rotate along one axis within the cup 802 or
the cup insert 804 may be constrained with some stopping and/or
locking mechanism. This stopping and/or locking mechanism may constrain the
translation in either direction and allow differing amounts of translation for
each patient, depending on the amount of translation needed for each patient.
Referring to FIGS. 39 and 40, a second exemplary mobile
bearing acetabular component 840 comprises an acetabular
cup 842 and a repositionable cup insert 844.
The repositionable cup insert 844 includes a semicircular
rib 846 having a dove tail cross-section that extends
circumferentially on the cup insert's exterior surface. This rib 846 is
adapted to be at least partially received within a
corresponding semicircular groove 848 formed on the interior of
a semicircular track (not shown). In this exemplary embodiment,
the groove 848 takes on a dove tail shape. It should be noted,
however, that other rib 846 and groove 848 shapes may
be utilized such as, without limitation, the rib 846 having a
T-shape and the groove 848 having a corresponding cavity to
receive and retain the rib. Moreover, it is within the scope of the disclosure
for the rib 846 to be part of the track, while
the groove 848 is located circumferentially on the cup
insert 844.
Another way for this implant to achieve translation in two
directions is to include an intermediate liner 851 (see FIG. 39)
that fits between the acetabular cup 842 and the insert
liner 844. This intermediate liner 851 can be polyethylene,
metal, ceramic or any other bearing surface material. The intermediate
liner 851 allows for the insert liner 844 to translate
along one direction with respect to the intermediate liner, while
the intermediate liner 844 translates along a second direction
(e.g. perpendicular to the first direction) within the acetabular
cup 842.
In this exemplary embodiment, the liner 851 includes
a projection 852 formed on its circumferential exterior that is
received within a corresponding semicircular groove 854 formed
on the interior of the cup 842. In this exemplary embodiment,
the groove 854 takes on a dove tail shape. It should be noted,
however, that other projection 852 and groove 854 shapes
may be utilized such as, without limitation, the projection 852 having
a T-shape and the groove 854 having a corresponding cavity to
receive and retain the projection. Moreover, it is within the scope of the
disclosure for the projection 852 to be located on the interior
of the acetabular cup 842, while the groove 854 is
located on the circumferential exterior of the track 850.
The semicircular track 850 in FIG. 39 is
rotationally offset ninety degrees from the groove 854 on the
interior of the circumferential cup 842. It should be noted, however,
that the track 850 need not be offset to precisely ninety degrees and
may be offset at a variety of angles. In the manner shown in FIG. 39,
the cup insert 844 may slide toward an east or west direction
with respect to the semicircular track 850 and with respect to
the acetabular cup 842, thereby sliding the cup insert in an east or
west arcuate direction. At the same time, the track 850 may slide
toward a north or south direction with respect to the acetabular cup 842,
thereby sliding the cup insert 844 in a north or south arcuate
direction. In other words, the net result is that there are two degrees of
freedom for net movement of the cup insert 844 with respect to
the cup 842. A first degree of freedom is an arcuate motion in a
north or south direction, and the second degree of freedom is an arcuate motion
in an east or west direction, where the degrees of freedom are independent of
one another any may be exercised individually or in tandem.
Referring to FIG. 41, a third exemplary mobile
bearing acetabular component 870 comprises an acetabular
cup 872 and a repositionable cup insert 874.
The repositionable cup insert 874 includes a semicircular
rib 876 having a dove tail cross-section that extends
circumferentially on the cup insert's exterior surface. This rib 876 is
adapted to be at least partially received within a corresponding semicircular
groove 878 formed on the interior of a semicircular track 880.
In this exemplary embodiment, the groove 878 takes on a dove tail
shape. It should be noted, however, that other rib 876 and
groove 878 shapes may be utilized such as, without limitation,
the rib 876 having a T-shape and the groove 878 having
a corresponding cavity to receive and retain the rib. Moreover, it is within
the scope of the disclosure for the rib 876 to be part of the
track 880, while the groove 878 is located circumferentially on
the cup insert 874.
In this exemplary embodiment, the semicircular track 880 includes
a projection 882 formed on its circumferential exterior that is
received within a corresponding semicircular groove 884 formed on the
interior of the cup 872. In this exemplary embodiment, the
groove 884 takes on a dove tail shape. It should be noted, however,
that other projection 882 and groove 884 shapes may be
utilized such as, without limitation, the projection 882 having a
T-shape and the groove 884 having a corresponding cavity to receive
and retain the projection. Moreover, it is within the scope of the disclosure
for the projection 882 to be located on the interior of
the acetabular cup 872, while the groove 854 is
located on the circumferential exterior of the track 850.
The semicircular track 880 is rotationally
repositionable with respect to the groove 884 on the interior of
the circumferential cup 872. In this manner, the cup
insert 874 may slide toward an east or west direction with respect to
the semicircular track 880 and with respect to the acetabular
cup 872, thereby sliding the cup insert in an east or west arcuate
direction. In addition, the cup insert 874 is rotatable with
respect to the acetabular cup 872 in 360 degrees. At the same
time, the track 880 may slide toward a north or south direction with
respect to the acetabular cup 872, thereby sliding the cup
insert 874 in a north or south arcuate direction. In other words, the
net result is that there are three degrees of freedom for net movement of
the cup insert 874 with respect to the cup 872. A
first degree of freedom is an arcuate motion in a north or south direction, a
second degree of freedom is an arcuate motion in an east or west direction, and
a third degree of freedom is axial rotation, where the degrees of freedom are
independent of one another any may be exercised individually, at once, or in
tandem.
Another manner for this implant 870 to
achieve translation and rotation could be through the use of
an intermediate liner 890 that rotates freely with respect to
the acetabular cup 872, but includes a groove 892 that
allows the cup insert 874 to freely translate along one
direction. Since the intermediate liner 890 can freely rotate,
the direction of the insert liner translation may be in any direction with
respect to the acetabular cup 872 and/or the patient's natural
anatomy.
Although most THA acetabular cups are designed to be a
sphere, the cup may alternatively be elliptical allowing for the insert to
translate and/or rotate to a greater amount, if deemed necessary.
Referring to FIG. 42, an exemplary process for use with
hip joint surgery includes removing the native femoral head 900 of
a patient to reshape the neck of the femoral neck bone. After the femoral head
is removed, an implanted femoral head is inserted into and/or around the
reshaped femoral neck bone, resembling an implanted hip stem and femoral head.
While the shaped femoral head 900′ is shown as having a cylindrical
shape, it should be understood that the femoral head may be shaped in a
rectangular, spherical, cylindrical, trapezoidal, or another other shape that
could be beneficial for attaching a femoral ball 902 to the
native bone. Although not shown in this figure, the femoral head may have a
stem that inserts into the femoral neck bone. Also, the full femoral head bone
anatomy does not have to be removed and the implanted femoral head may be
attached onto the femoral head bone.
Referencing FIG. 43, another exemplary process for use
with hip joint surgery includes removing or reshaping the native femoral
head 900 of a patient to resemble the neck of a prosthetic femoral
component. Thereafter, a metal, ceramic or any other implantable
material cylindrical sleeve 910 is positioned to circumscribe
the reshaped bone 900′ (see FIG. 42) for stabilization. After
the sleeve 910 is mounted to the reshaped femoral
head 900′, a prosthetic femoral ball 902 is mounted to the
reshaped femoral head and/or femoral neck.
Referring to FIG. 44, a further exemplary process for
use with hip joint surgery includes removing and/or reshaping the native
femoral head and/or neck 900 of a patient to resemble the neck
of a prosthetic femoral component. In this exemplary process, the femoral
head 900 is reshaped and a trapezoidal sleeve 920 is
positioned to circumscribe the reshaped bone for stabilization. After
the sleeve 920 is mounted to the reshaped femoral head, a
prosthetic femoral ball 922 is mounted to the reshaped femoral head.
In the foregoing exemplary processes of FIGS. 42 and
43, it is also within the scope of the disclosure that the sleeve 910, 920 and
the femoral ball 902, 922 comprise a single, integral
component. Likewise, the femoral head can slide over the bone and/or the
femoral sleeve, or it can also have an internal like stem that could go into
the bone of the femur. It should also be noted that while the femoral head and
femoral sleeve are shown as two separate pieces, the femoral head and femoral
sleeve may also be one piece and/or modular to allow for these aspects to be
attached creating one secure piece.
Referring to FIGS. 45-49, a surgeon improperly reaming
out the acetabulum may lead to the placement of the acetabular cup at a
location not allowing for concentricity with the femoral head of the femoral
implant. Since concentricity of these components is essential to minimize or
eliminate both hip dislocation and/or femoral head separation, an irregular
acetabular cup or cup insert may be required to accommodate for the improper
reaming. Specifically, the exemplary irregular acetabular cup or cup
insert 1000 does not have uniformity in wall thickness, radius and/or
shape. This non-uniformity of either the acetabular cup and/or cup insert
allows for spherical center realignment to ensure concentricity between the
acetabular cup and femoral head centers.
Referring to FIG. 45, present day acetabular
cups 1100 all have a uniform wall thickness.
Referencing FIGS. 46-48, if a surgeon reams out too
much bone from one or more regions (e.g., points A-E in FIG. 46) of the
acetabulum, then the surgeon can use a non-uniform acetabular cup
and/or cup insert 1000, 1002, 1004. The non-uniform
acetabular cup and/or cup insert 1000, 1002, 1004 has
a non-uniform shape and wall thickness. The non-uniformity is used to shift the
spherical center in various directions, such as, without limitation, proximal,
distal, medial, and lateral. In this manner, the spherical center of the
femoral component is aligned with the spherical center of the acetabular
component.
Referring to FIG. 49, the surgeon who reams out too
much bone from the acetabulum may need a spacer 1010 that allows
for the center of the acetabular cup or cup insert 1100 to be
repositioned into alignment. This spacer 1010 may be mounted to
the front side or back side of the acetabular cup and/or cup insert 1100.
And the spacer 1010 may have a uniform of non-uniform wall
thickness and shape. The spacer 1010 may be of any shape
necessary to shift the spherical center in the proper direction. Exemplary
materials for fabricating the spacer 1010 include, without
limitation, titanium, cobalt chromium, high density polyethylene, and stainless
steel.
Referencing FIGS. 46-49, if the surgeon reams away too
much bone in the region between points A and B, B and C, C and D or A and D
of FIG. 46, the surgeon may use either a non-uniform acetabular cup
or cup insert 1000, 1002, 1004 or a spacer 1010 to
shift the acetabular cup spherical center to the desired direction. It is also
within the scope of the disclosure to use spacers 1010 with one
or more of the non-uniform acetabular cup or cup insert 1000, 1002, 1004 to
further shift the acetabular cup spherical center to the desired direction.
Though the above exemplary embodiments have all been
discussed with respect to the hip joint, it is also within the scope of the
disclosure to apply these same principles to other joints of the body
including, without limitation, shoulder joint, elbow joint, and ankle joint. In
other words, the shoulder joint, elbow joint, and ankle joint may be inserted wherever
the foregoing describes a hip joint. And those skilled in the art should thus
understand that the teachings and embodiments are equally applicable to
shoulder joints, elbow joints, ankle joints, and hip joints.
Following from the above description and invention
summaries, it should be apparent to those of ordinary skill in the art that,
while the methods and apparatuses herein described constitute exemplary
embodiments of the present invention, the invention contained herein is not
limited to this precise embodiment and that changes may be made to such
embodiments without departing from the scope of the invention as defined by the
claims. Additionally, it is to be understood that the invention is defined by
the claims and it is not intended that any limitations or elements describing
the exemplary embodiments set forth herein are to be incorporated into the
interpretation of any claim element unless such limitation or element is
explicitly stated. Likewise, it is to be understood that it is not necessary to
meet any or all of the identified advantages or objects of the invention
disclosed herein in order to fall within the scope of any claims, since the
invention is defined by the claims and since inherent and/or unforeseen
advantages of the present invention may exist even though they may not have
been explicitly discussed herein.
What is
claimed is:
1. A
prosthetic hip joint comprising:
a femoral
component including a femoral head; and,
an
acetabular component including an acetabular cup and an acetabular cup insert,
the acetabular cup insert sized to receive the femoral head;
wherein the
femoral head includes a spherical center that matches a spherical center of a
patient's native femoral head;
wherein the
acetabular component includes a cavity with a spherical center that matches a
spherical center of a cavity of a patient's native acetabulum; and,
wherein the
femoral head center of the femoral component is concentric with the center of
the cavity of the acetabular component.
2. The
prosthetic hip joint of claim 1, wherein a radial thickness of the
acetabular cup is nonuniform along a circumferential length.
3. The
prosthetic hip joint of claim 1, wherein a radial thickness of the femoral
head is nonuniform along a circumferential length.
4. The
prosthetic hip, joint of claim 1, wherein an outer aspect of the
acetabular cup is nonspherical and an inner aspect of the acetabular cup is
spherical.
5. The
prosthetic hip joint of claim 1, wherein an outer aspect of the acetabular
cup is spherical and an inner aspect of the acetabular cup is nonspherical.
6. The
prosthetic hip joint of claim 1, wherein the acetabular component is
configured to be secured to the patient such that the spherical center of the
cavity of acetabular component is concentric with the spherical center of the
cavity of the patient's native acetabulum.
7. A
prosthetic hip joint comprising:
a femoral
component including a femoral head; and,
an
acetabular component including an acetabular cup and an acetabular cup insert,
the acetabular cup insert sized to receive the femoral head;
wherein the
femoral head includes a spherical center that matches a spherical center of the
acetabular cup insert;
wherein the
acetabular cup insert includes a cavity with a spherical center that matches a
spherical center of a cavity of a patient's native acetabulum, the spherical
center of the acetabular cup insert being concentric with (i) the spherical
center of the femoral head and (ii) the spherical center of the cavity of the
patient's native acetabulum when the femoral component and the acetabular
component are implanted.
8. The
prosthetic hip joint of claim 7, wherein a radial thickness of the
acetabular cup is nonuniform along a circumferential length.
9. The
prosthetic hip joint of claim 7 wherein a radial thickness of the
femoral head is nonuniform along a circumferential length.
10. The
prosthetic hip joint of claim 7 wherein an outer aspect of the
acetabular cup is nonspherical and an inner aspect of the acetabular cup is
spherical.
11. The
prosthetic hip joint of claim 7, wherein an outer aspect of the acetabular
cup is spherical and an inner aspect of the acetabular cup is nonspherical.
External links
Komistek RD. Maintaining proper mechanics THA. US20120221115A1 February 24, 2011. 2012. patents.google
Publications
of invention
AU2012249145 (A1)
AU2012249145 (B2)
AU2016200496 (A1)
AU2016200496 (B2)
AU2018226451 (A1)
CN103796616 (A)
CN103796616 (B)
CN105232189 (A)
CN105232189 (B)
EP2677967 (A2)
EP2677967 (A4)
EP3329881 (A1)
EP3329881 (B1)
EP3533418 (A1)
EP3673874 (A1)
JP2014517703 (А)
JP2016179229 (A)
JP5956474 (B2)
JP6211649 (B2)
US10064729 (B2)
US2012221115 (A1)
US2015305872 (A1)
US2017027704 (A1)
US2019008646 (A1)
US9023112 (B2)
WO2012148544 (A2)
WO2012148544 (A3)
ZA201307148 (B)
2012KomistekRD
Authors & Affiliations
Richard D.
Komistek, Knoxville, TN (US)
Keywords
ligamentum capitis femoris, ligamentum teres, ligament
of head of femur, endoprosthesis, prosthesis,
invention, bipolar, total
NB! Fair practice / use: copied for the purposes of criticism, review, comment, research and private study in accordance with Copyright Laws of the US: 17 U.S.C. §107; Copyright Law of the EU: Dir. 2001/29/EC, art.5/3a,d; Copyright Law of the RU: ГК РФ ст.1274/1.1-2,7


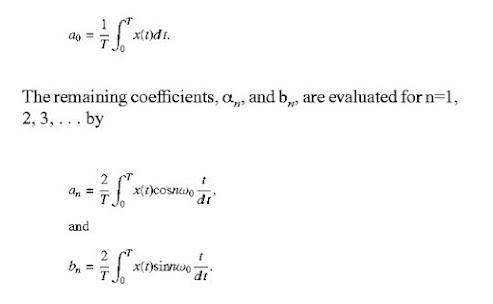













Comments
Post a Comment