Invention (Patent Application Publication): Sporring
SL, Olsen O, Lauritzen JB, Andersen TL, Brondsted P, Bechgaard K, Hansen JG,
Jensen H, Almdal K, Mouritsen S. Medical device for insertion into a joint, WO2006133711A2 (2006).
WO2006133711A2 Denmark
Worldwide applications 2006 WO EP 2008 US
Application PCT/DK2006/000343 events:
2006-06-14 Application filed by Cartificial A/S
2006-06-14 Priority to EP06753312A
2006-12-21 Publication of WO2006133711A2
2007-06-14 Priority to PCT/DK2007/000290
2007-09-20 Publication of WO2006133711A3
2008-12-09 Priority to US12/331,335
Medical device for insertion into
a joint
Sune Lund
Sporring, Ole Olsen, Jes Bruun Lauritzen, Tom Løgstrup Anderson, Povl Brøndsted,
Klaus Bechgaard, Jan Guldberg Hansen, Henrik Jensen, Kristoffer Almdal, Søren Mouritsen
Abstract
The present invention relates to implants used for
alleviating and/or preventing conditions relating to damaged joints involving
articulating surfaces. The implants com- prise fibre of polymer and/or metal,
and can be used as an artificial joint, as part of an artificial joint or as an
artificial joint spacer made to replace the missing cartilage or to improve the
slidability between two natural and/or artificial components of the body, or
between a natural and artificial component. The product of the invention can be
used to partly or entirely coat medical products or to make up implants partly
or entirely.
Description
Medical
device for insertion into a joint
Field of invention
The present invention relates to a method and a medical
device for alleviating and/or preventing conditions relating to damaged joints
involving articulating surfaces. Especially the present invention relates to
medical products which are coated partly or entirely by one or more polymeric
components and to medical products which comprises metal and polymeric
materials. The product of the present invention can be used as an artificial
joint, as part of an artificial joint or as an artificial joint spacer made to
replace the missing cartilage or to improve the slidability between two natural
and/or artificial components of the body, or between a natural and artificial
component of the body. All patent and non-patent references cited in the
application, are also hereby incorporated by reference in their entirety.
Background of invention
At present, joint damage, such as cartilage damage, is
treated by replacing the joint with an artificial joint. However, serious
complications are caused by the replacement of artificial joints, in particular
a high occurrence rate of loosening problems resulting in breakage of the bones
around the artificial joint. In the case of cartilage damage a repair with
cartilage substitution placed into intact bones is to be preferred instead of
replacing the entire joint.
In particular, the invasive character of the fixation of the
prostheses such as anchoring of the prosthesis with screws and pins results in
numerous side-effects such as risk of infection, loosening as mentioned above,
damage on excising bone due to interruption of blood supply and necrosis.
A device for replacement within a joint should preferably
enable the normal function and movements of the joint. Weight-bearing joints,
in which movement in more than one direction takes place, are normally rather
difficult to replace. A prosthetic device should enable the normal movement of
the joint. During walking, the normal movement of for example the hip joint
corresponds to about 37°-41 ° flexion/extension, 2°-14° adduction/abduction and
a rotation of about 2°-16°. During movement from standing to sitting position a
flexion of hip joint corresponds to a movement from 0 to 90 degrees. When
studying the movement of femoral caput to the acetabulum the latter movement
includes a rotation of 90 degrees.
Many medical devices are implanted into load-bearing joints
such as knees, hips, etc, or utilised in the human body where mechanical
function provide high strength or shape stability such as heart valves, breast
prosthesis, stent, catheter, etc. As such, these medical devices must be very
strong and possess a high degree of wear resistance. Prosthetic medical devices
manufacturers constantly work toward developing better products by improving
their physical properties. Improved wear resistance, for example, is a
desirable quality to impart to a prosthetic medical device. Improving wear
resistance without losing strength or causing oxidative degradation is a
difficult balance to obtain.
A need for improved prosthetic medical devices with improved
wear resistance exists.
Many implants produce small particles on the surface of the
implant when subjected to motion. The particles are liberated from the implant
as abrasion caused by the motion of the implant and friction towards other
components within the body. These particles can give rise to inflammation
within the individual where the implant is implanted. In a test controlling the
wear characteristic of a hip-joint implant with traditionally polyethylene (PE)
acetabular cups, about 1012 particles are produced pr. million of
movements (cycles).
The use of ceramic or cross-linked PE cups slightly reduces
the number of particles produced. Still the produced particles give rise to
inflammation and also there is a risk that the implant becomes loose.
An implants produced with a smooth UHMWPE coating according
to the present invention will only produce 5*108 or less particles pr.
million cycles. Thus a lesser degree of inflammation and a longer life of these
implants are some of the results when implanted into an individual.
Summary of invention
The present invention relates to medical devices which may
be used in damaged joints involving articulating surfaces.
The medical device may comprise at least one fabric of one
or more polymer fibre and/or of one or more metal fibre and/or a 3D network of
polymer fibre and/or metal fibre. The at least one fabric may also be at least
one 2D network. Fabrics, 2D networks and 3D networks may be used together in
one medical device.
The volume within the fibres making up the device may be
filled up with one or more polymer optionally further comprising one or more
metal components or the volume is filled with a metal or metal alloy. The
volume to be filled up with one or more polymer optionally further comprising
one or more metal components can be determined according to the shape and total
volume of the final device ready to use. The device may be subjected to
size-reducing processes during preparation, thus the initially volume defined
by the fibres of the fabrics and/or networks can be larger than the
corresponding volume within the final device.
Biocompatible polymers and biocompatible metals can be used
to make up the device. Polymers and metals are described elsewhere herein.
A device or part of a device made up according to the
present invention may be used for any implant. Especially implants to be
positioned into joints can be made, such as an acetabular cup, a spacer to be
located between an acetabular cup and a head of hip stem, or an interpositional
arthroplasty. The head of the hip stem can be made of metal or a ceramic
material. The device may also be a cup shaped spacer which is positioned
between the natural femoral stem or a metal femoral stem and a natural
acetabular cup or a prosthetic acetabular cup.
The medical may comprise at least a first surface area,
wherein at least a first poly- meric component optionally further comprising
one or more metal components is attached to or coated on the first surface
area. The material to coat a medical implant can be made with fabrics, 2D
networks and/or 3D networks of polymer fibre and/or metal fibre where the fibre
are connected to each other or with another polymer and/or metal material as
described herein.
A medical device produced from the materials described
herein as well as devices produced by other methods may be coated or covered
partly or entirely with a polymeric material of the present invention, the
polymeric material optionally comprises a metal component.
The medical device may have an upper surface, a lower
surface and at least one edge and wherein at least the one edge may be sealed
by a collar. The collar can be made of a material according to the present
invention, e.g. it can be made with fabrics, 2D networks and/or 3D networks of
polymer fibre and/or metal fibre where the fibre are connected to each other or
with another polymer and/or metal material as described herein.
Also the collar may be made of a first, second and/or a
third polymeric component optionally further comprising one or more metal
components.
A surface of the device can be made smooth by using the
material according to the present invention. The device comprises at least a
first and a second side, wherein the at least first side is made of a first
polymeric component optionally further comprising one or more metal components
and wherein the part of or the entire of the first and/or second side has a
frictional resistance of less than 0.5 Newton.
The smooth surface can be made by using a material according
to the present invention, e.g. it can be made with fabrics, 2D networks and/or
3D networks of polymer fibre and/or metal fibre where the fibre are connected
to each other or with an- other polymer and/or metal material as described herein.
The smooth surface may further include one or more cavities.
The device may have at least one through-going perforation
which is not for liga- ments. The perforation may have a diameter of at least
0.01 mm and can conduct liquid from one first side of the device to another
second side of the device. The liquid may be synovial fluid or a physiological
salt solution and/or another biocompatible liquid.
The device may also be equipped with an inner volume between
the first and second side and where said liquid can be within this volume. This
inner volume may further comprise a network of at least one polymeric and/or
metal component, e.g. polymeric fibres and/or metal fibres.
The surface of a medical device according to the present
invention may be a self- healing surface. The medical device comprises a first
polymeric component optionally further comprising one or more metal components
which may be self-healing when subjected to injury before implantation, during
implantation and/or after implantation.
The self-healing surface can be made by using a material
according to the present invention, e.g. it can be made with fabrics, 2D
networks and/or 3D networks of polymer fibre and/or metal fibre where the fibre
are connected to each other or with another polymer and/or metal material as
described herein. Other polymers described herein can also be used to produce a
self-healing surface.
The surface material may be any material described herein,
e.g. a composite polymeric component optionally further comprising one or more
metal components. The device can include a microencapsulated healing agent that
is released upon injuries hereby polymerization of the polymeric component is
triggered by contact with an embedded catalyst/initiator. The healing agent can
be monomers of the first polymeric component and/or of a second polymeric
component.
The medical device according to the present invention may be
two parts or units which fit into each other, the device comprise at least a
first unit with at least a convex surface and a second unit with at least a
concave surface, where the convex and concave surface is congruent with each
other and the first unit fit partially or entirely into the second unit, and
the first and/or second unit comprises at least one polymeric component
optionally further comprising one or more metal components. Each unit can be
made by using a material according to the present invention, e.g. it can be
made with fabrics, 2D networks and/or 3D networks of polymer fibre and/or metal
fibre where the fibre are connected to each other or with another polymer
and/or metal material as described herein.
The first
and second unit can have substantially similar sizes, or be of different sizes.
The units may be movable when compared to each other, and the units can
continue to be in contact during a movement of the body and/or return to be in
contact when a movement is finished.
The medical device according to the present invention may
comprise at least an upper layer, a first middle layer and a lower layer, wherein
the upper layer and said lower layer is made from one uninterrupted piece of at
least a first polymeric component optionally further comprising one or more
metal components.
The material to make up the device may be a fabric, 2D
network and/or 3D network which can be constructed in one piece, which is
folded around an axis and hereby forming the upper and lower layer.
The upper and lower layer of the device may be similar or
substantially similar in size, and may be formed from a tube folded around the
axis. The tube may have a uniform diameter along the length, or may contract in
a first and/or a second end of said tube, e.g. the tube contract in the axis.
The device may in the axis further com- pris at least one aperture.
The medical device according to the present invention may
also be thicker at the periphery of the device. The device may comprise at
least a first polymeric component optionally further comprising one or more
metal components, wherein the device has a middle area which at least in one
dimension is surrounded by an outer area, and wherein the thickness of the
outer area at least partly is larger than the thickness of the middle area, and
the outer area ends in at least one edge of said device. The device may have
any suitable shapes, e.g. cup-shaped or approximately cup- shaped, and wherein
a middle area is the top and an outer area is a skirt. The skirt may be thicker
that the top.
The device may also be a layered device. The device in a
layered structure may comprise
• at least one upper layer of said first polymeric component
optionally further comprising one or more metal components,
• a middle layer of said second polymeric component
optionally further comprising one or more metal components, and
• at least one lower layer of said third polymeric component
optionally further comprising one or more metal components, where the chain
length of the first polymeric component and the third polymeric component is
longer than the chain length of the second polymeric component.
In a layered device as described above, each layer may
itself be composed of at least two sub-layers of polymeric fabric and/or a 2D
network and/or a 3D network, each sub-layer optionally further comprising one
or more metal components constructed of the polymeric components described
herein, and at least one layer of polymeric film optionally further comprising
one or more metal components, said polymeric film constitutes a layer between
the two layers of the polymeric fabrics and/or 2D network and/or 3D network.
Also the middle layer, the film may further comprise one or
more metal components.
The metal used in the device may be one or more metals
selected from the group of metal and metal alloys of titanium, gold, silver,
chromium-cobaltum, zirconia, cobalt- chromium-molobdenum alloy and Stainless
Steel alloys and/or a ceramic of one or more of these metals and alloys. Other
metals may also be included e.g. in the alloys.
The polymers and metals including alloys used may be in the
form of e.g. a powder, granulate, chopped fibres, long fibres, 2D structural
components like plates, 3D structural components like shaped plates or
hemicircles with holes. Also a combination of these forms may be used. Instead
of using fabrics, 2D networks and/or 3D networks of polymer fibre and/or metal
fibre to produce the devices as described above, it may also be possible to use
polymers and/or metals which are suitable for injection moulding, these
materials may further include components of polymer and/or metal, as described
herein.
Description of drawings
Fig. 1 illustrates a longitudinal section of a cup-shaped
medical device.
Fig. 2 illustrates a cross-section of a cup-shaped medical
device.
Fig. 3 illustrates a longitudinal section of a cup-shaped
medical device. The device has a shirt in which the material is thicker than
the material at the top of the device.
Fig. 4 illustrates a cup-shaped medical device. The line
denoted "E" illustrates equator, which is the border between the top
"T" and the shirt "S".
Fig. 5
illustrates a cup-shaped medical device (2) with a collar (1).
Fig. 6 illustrates a longitudinal section of a cup-shaped
medical device. The device has a shirt in which the material is thicker than
the material at the top of the device. Furthermore the device has a collar with
a marker.
Fig. 7 illustrates the edge of the medical device in Fig. 6.
The edge is enclosed by a collar, and this collar supports a marker (3).
Detailed description of the invention
The present invention provides a desirable balance of
improved wear resistance and high tensile strength and toughness in the
polymeric compositions used for medical devices. The implants may also include
metal e.g. as a composite with polymeric materials. It has been discovered that
wear resistance can be improved without sacrificing other desirable properties
such as toughness or strength by controlling the amount of different polymeric
and/or metal substrate comprising the prosthetic device. The products of the
invention has a high tensile strength and improved wear resistance as well as
the capability to absorb shocks, impacts and pressure load, also it reduces the
amount of tearing off.
The medical device may be designed to occupy the pelvis cup;
to occupy at least part of the intra-articular cavity to partly or completely
fill the role of natural cartilage within a joint; and/or to be an interpositional
arthroplasty. The devices or their units may be designed so as to occupy the
whole of the cavity or merely a portion of the intra-articular cavity, such as
the portion of the cavity where cartilage is worn or where much of the pressure
is exerted.
The medical devices may also be designed to replace at least
part of a bone within a joint together with the intra-articular cavity to
partly or completely fill the role of the bone which is replaced and to partly
or completely fill the role of natural cartilage within a joint.
The material for the medical devices is primary polymers
although other materials as described elsewhere herein can be used. The other
materials e.g. metals may be used together with the polymers.
In an aspect of the invention a medical device comprises at
least one fabric of one or more polymer fibre and/or of one or more metal fibre
and/or a 3D network of polymer fibre and/or metal fibre. The polymer fibre and
metal fibre which may be used are described elsewhere herein.
In an embodiment the device comprises an area at least
defined by the outermost fibres of the network, this area is filled up with one
or more polymer optionally further comprising one or more metal components or
said area is filled with a metal or metal alloy. The polymer and metal
components used may be anyone described elsewhere herein. The fibres of the
network may during the production process of the device be located
substantially in the position where the fibres will be located in the final
device. Also the fibres making up the network may during the production process
be located in different positions when compared to the location of these fibres
in the final device. Hereby the volume to be filled up may be defined by the
outermost fibres of the network and is a volume defined by the corresponding
volume of the network in the final device, hereby the volume defined by the
network of fibres is larger in the production process than the volume defined
by the final device.
In the production process heat and/or pressure may be used
to connect polymers and/or metals in different forms to each other.
In an embodiment the polymer fibre and/or the polymer
optionally further comprising one or more metal components may be a polyolefin,
such as polyethylene, such as UHMWPE. Also other polymers as described
elsewhere herein may be used. The polymer fibre and the polymer optionally
further comprising one or more metal com- ponents can be different types of
polymers. Also the metal fibre and the metal components can be different types
of metal.
In an aspect of the invention a medical device comprises at
least a first surface area, wherein at least a first polymeric component is
attached to the first surface area.
In an embodiment of the device, the first surface area is a
part or the entire surface area of a first volume, where the first volume is
smaller than the final volume and where the final volume is the total volume of
the device before implantation, and wherein the difference between the final
volume and the first volume is a volume made up by at least a first polymeric
component, a first metal component or a combination of a first polymeric
component and a first metal component.
At least a first polymeric component and/or a first
polymeric component together with a first metal component can coat a part or
the entire surface of an implant. A second and/or third polymeric component or
a combination of a second and/or third polymeric component together with a
second and/or third metal component may coat the same surface part as coated by
the first polymeric component, optionally together with a first metal
component, or the second and/or third polymeric compo- nent, optionally
together with a second and/or third metal component, may coat a part of the
surface or different parts of the surface than coated by the first polymeric
component optionally with a first metal component.
The term "first volume" is used to describe the
produced implant or part of an im- plant at a stage in the production where
only a coating according to this invention is lacking on the implant. This
definition does not exclude the possibility of treating the coated implant e.g.
by irradiation or by a liquid solution and thus the final volume being the
coated implant slightly can change in volume.
In an embodiment the first volume has a shape which
corresponds to the final volume of the device before implantation. In this case
"corresponds to" can mean having exactly the same shape just with a
smaller volume, or can mean that the overall shape is similar when comparing
the first volume and the final volume, but in one or more zones the two volumes
correspond to a lesser degree to each other than in other zones.
In a further embodiment the first volume corresponds to at
least 50% of the final volume, such as at least 60%, such as at least 70%, such
as at least 80%, such as at least 85%, such as at least 90%, such as at least
95%, such as at least 97%, such as at least 99%. This calculation can be made
based on the overall shape of the first and final volume or can be based on a
comparison between different although related zones of the first and final
volume of the implant.
In an embodiment the first volume corresponds to a lesser
degree to the final vol- ume in areas where the at least first polymeric
component optionally together with a first metal component, is attached. If
only a part of the surface of the implant is to be coated with the first
polymeric component and/or second polymeric component and/or third polymeric
component, optionally together with a first, second and/or third metal
component, respectively, the zones including the surface area to be coated will
correspond to a lesser degree to the final volume of the implant than zones
located elsewhere in the implant.
In an embodiment the first volume further comprises
different first zones, and where the different first zones are smaller volumes
of the first volume and the first volume in different first zones corresponds
to a different degree to related final zones of the final volume, and where the
final zones are smaller volumes of the final volume. The zones of the first
volume and final volume are not of a specific volume, but are to be determined
from implant to implant. When a zone of a first volume corresponds to a zone of
a final volume, this means the two zones are located in similar areas of the
implant when taking into account that the final volume is larger than the first
volume.
In an embodiment the volume of the first zones corresponds
to at least 50% of the volume of the related final zones, such as at least 60%,
such as at least 70%, such as at least 80%, such as at least 85%, such as at
least 90%, such as at least 95%, such as at least 97%, such as at least 99%.
In another embodiment the volume of the first zones
corresponds to a different degree to the volume of the related final zones and
where the different degree is selected between 50-100%, such as 60-100%, such
as 70-100%, such as 80-100%, such as 90-100%, such as 95-100%, such as 50-90%,
such as 60-90%, such as 70- 90%, such as 80-90%, such as 50-80%, such as
60-80%, such as 70-80%, such as 50-70%, such as 60-70%. Some zones of the first
volume may be relatively smaller than other zones when compared to the related
zones of the final volume.
In another embodiment the first volume further correspond to
the final shape of the final volume. Only slight differences exist between the
shape of the first volume and the shape of the final volume.
In an embodiment the first surface area comprises the outer
surface of one or more of the first zones. The number of zones constituting the
first surface area can be calculated if a zone is defined as a volume of the
implant where the zone(s) next to this zone have or is going to have a coating
that differs in thickness or overall polymeric composition when compared to the
zone of interest.
In an embodiment the at least first polymeric component is
attached to the entire outer surface of the first volume. An implant can be
coated over the entire outer surface with the first polymeric component,
optionally together with a first metal component and/or a second polymeric
component, optionally together with a second metal component and/or a third
polymeric component, optionally together with a third metal component. In an
embodiment the device at least in the first area is subjected to wear when the
device is located in an individual. An implant can be coated either in only the
area(s) which are subjected to wear or also in other areas. The polymeric
components, op- tionally together with metal components, used as coating are
described elsewhere herein and can be one or more polymeric components
optionally together with one or more metal components.
In an embodiment the first volume of the device may be made
partly or entirely of polymer, bone and/or metal. The implant to be coated can
be made of any material which can be coated by one or more polymeric
components, optionally together with metal components, as described elsewhere
herein.
In implants composed of different units, one or more of
these units can be coated partly or entirely by the polymeric components
described herein, the polymeric components may comprises metal components as
also described elsewhere herein.
In an embodiment the first polymeric component, optionally
together with one or more metal components, constitute at least 1% of the
entire outer surface of the device, such as at least 3%, such as at least 5%,
such as at least 8%, such as at least 10%, such as at least 15%, such as at
least 20%, such as at least 25%, such as at least 30%, such as at least 35%,
such as at least 40%, such as at least 50%, such as at least 60%, such as at
least 70%, such as at least 80%, such as at least 90%, such as at least 99%.
This calculation is based on a comparison of the outer area of the first volume
and the outer area of the final volume.
In another embodiment the first polymeric component
constitute at least 1% of the diameter of the final volume of the device, such
as at least 3%, such as at least 5%, such as at least 8%, such as at least 10%,
such as at least 15%, such as at least 20%, such as at least 25%, such as at
least 30%, such as at least 35%, such as at least 40%, such as at least 50%,
such as at least 60%. This calculation is based on a comparison of the diameter
of the first volume and the diameter of the final volume, and can be an overall
calculation or a calculation within one or more zones. In an embodiment the
first polymeric component, optionally together with metal components, may be
located at the outside of the device in areas that is subjected to wear when
located in a body. The implant can be coated only in areas that is subjected to
wear, or in other areas too. It is important to coat the implant in the areas
subjected to wear.
In an
embodiment the first polymeric component, optionally together with metal
components, is in the form of a fabric. The first, second and third
component, optionally together with metal components, can be in any form described
herein and can be combined in any way as described elsewhere herein.
In an embodiment the first and the second polymeric
component comprises the same monomeric component or the same components of the
composite material, and the first and second polymeric components are
differently crystallized within the implant ready to implant. The first and the
second polymeric component may comprise a first and/or second metal component.
These metal components may be similar or different types of metal or the same
type of metal although in pieces of different sizes e.g. short fibre and long
fibre.
Also the third polymeric component can be of the same
monomeric component or the same components of the composite material as the
first and/or second polymeric component, and/or be differently crystallized
within the implant ready to implant when compared to the first and/or second
polymeric components. The third polymeric component may comprise a third metal
component. This metal component may be of similar or different types of metal
than the first and/or second metal component, or the same type of metal
although in pieces of different sizes e.g. shorter fibre and/or longer fibre
In an embodiment the first and the second polymeric
component comprises the same monomeric component or the same components of the
composite material and the first and second polymeric components have different
morphology within the implant ready to implant.
Also the third polymeric component can be of the same
monomeric component or the same components of the composite material as the
first and/or second polymeric component and have a differently morphology
within the implant ready to implant when compared to the first and/or second
polymeric components.
In an embodiment the first polymeric component and/or the
second polymeric com- ponent and/or the third polymeric component, each
optionally comprising one or more metal components may be attached to the first
volume by ultrasound welding, laser welding, heating and/or gluing. Any method
to secure the polymeric components to the first volume can be used.
In an embodiment the first polymeric component and/or the
second polymeric component and/or the third polymeric component, each
optionally comprising one or more metal components are attached to each other
before attached to the first volume. The polymeric components, optionally
comprising one or more metal components, can be attached to each other e.g. in
a layered structure as described else- where herein, but also other
compositions described herein can be used.
In an embodiment the first polymeric component and/or the
second polymeric component and/or the third polymeric component, each
optionally comprising one or more metal components, are attached to the first
volume one polymeric component at a time. The polymeric components, optionally
comprising one or more metal components, can be attached to the first volume of
the implant in any order suitable for the implant, thus the first polymeric
component need not be the polymeric component situated closest to the first
volume. The number and order of the polymeric components, optionally comprising
one or more metal components, are not limited.
In an embodiment the number of layers of the polymeric
components, optionally comprising one or more metal components, includes more
than one layer of one of the polymeric components, optionally comprising one or
more metal components, and each of the layers are attached to the first volume
one polymeric component at a time. Preferred number of layers is described
elsewhere herein.
In an embodiment the said device comprises a part of or an
entire hip joint prosthesis with a natural or metal and/or ceramic femoral stem
articulating against an acetabular cup to be placed in the pelvis. The
acetabular cup may be a PE acetabu- lar cup, a UHMWPE acetabular cup or a metal
acetabular cup, and the metal or ce- ramie femoral stem and/or the acetabular
cup may be covered according to the possibilities described elsewhere herein.
In an aspect of the invention a device comprises an
acetabular cup or socket to be inserted in the pelvis cup, where the acetabular
cup may be made of any polymeric material and which may be covered and/or
reinforced with long polymeric fibre. The fibre may be in any form as described
elsewhere herein.
The long polymeric fibre which may cover and/or reinforce an
acetabular cup, may be in the form of one or more fabric or one and/or more
layers of fibre as described elsewhere herein or may be a 3D-structure. The
device comprising fabric and/or layers of fibre may be produced as described
elsewhere herein. In this context a 3D- structure is a composite made of a 3D
(three-dimensional) network of polymer fibre and/or metal fibre filled with and
optionally entirely surrounded by a polymeric com- ponent. The 3D-network may
have a knitted, crochet and/or weaved structure or may be made by any other
method described elsewhere herein. The polymeric component to fill out the 3D
network may be any polymer mentioned elsewhere herein.
Preferred is an acetabular cup as described above where the
long polymeric fibre are UHMWPE fibre, and the polymeric material to fill out
the space between the fibre has a melting point below the melting point of the
used UHMWPE fibre.
More preferred is an acetabular cup as described above which
may be covered with a fabric (2D-network) and/or a 3D-network of UHMWPE fibre,
and where the polymeric material to fill out the space between the fibre is a
polyethylene (PE) material. The PE material to fill out the space between the
fibre may be only one type of PE or may be PE of different length.
In an embodiment the acetabular cup as described above may
be used together with a cup-shaped spacer made in accordance with the
description elsewhere herein.
In an aspect of the invention the device is part of a hip
joint prosthesis or another prosthesis, and the device comprises a spacer
between two parts of a prosthesis or between a part of a prosthesis and a
natural bone of an individual. In a preferred embodiment a cup-shaped spacer is
positioned between the natural femoral stem or a metal femoral stem of a hip
joint prosthesis and a natural acetabular cup or a prosthetic acetabular cup.
In another embodiment the device is a spacer to be
positioned between two natural bone parts of an individual e.g. as an
interpositional arthroplasty.
In an aspect of the invention, the device includes at least
a first polymeric compo- nent optionally with a first metal component, and the
device has a middle area which at least in one dimension is surrounded by an
outer area, and wherein the thickness of the outer area at least partly is
larger than the thickness of the middle area, and the outer area ends in at
least one edge of the device.
In an embodiment the middle area of the device is surrounded
by the outer area in two dimensions. The device may have any shape suitable to
be allocated into a joint of a mammal. The device may be flat or substantially
flat. Furthermore the device may cup-shaped. Other shapes are described
elsewhere herein.
In another embodiment the device is homogenous in dimensions
around an axis, and the axis is a central axis according to one dimension of
the middle area. The axis indicates a line separating the device into two
substantially equally sized units.
In an embodiment, the device is heterogeneous in dimensions
around an axis, and the axis is an approximately central axis according to one
dimension of the middle area. The axis indicates a line separating the middle
area of the device into two substantially equally sized units. The outer area
is larger on one side of the device than on other sides of the device.
In an embodiment the middle area and the at least one edge
are in different planes. The device is non-homogeneous in shape and
non-homogenous with respect to the planes of the edges.
In an embodiment at least one edge are in different planes.
One edge is in one plane and at least another edge is in another edge, and when
these planes are pro- jected to a similar plane, this similar plane constitutes
an outline of the edge or edges of the device, and the outline has a shape that
is selected from a shape from triangular to circular.
The shape of the device may be any possible figure in each
dimension where the shape may constitute a surface being flat, curved, waved,
undulated, bent, bowed, crooked, while the overall shape of the device may be
but is not limited to circular, oval, triangle, squared, rectangle, cubed,
bowl, cup, crown, cap, basin, heart, egg, kidney, figure of eight, preferred
shape is cup or hemispherical. The thickness of the device may also vary, as
described elsewhere herein.
In an embodiment the device is cup-shaped or approximately
cup-shaped, and the middle area is a top and the outer area is a skirt.
In the cup-shaped device with a rounded top, a line separating
the top and the skirt is equator, and the outmost part of the skirt is the edge
of the device. Equator is located around the cup-shaped device where the
curvature of the cup changes to follow the tangent to the cup.
The cup-shaped or approximately cup-shaped device may have a
substantially uniform thickness all over the device or the device may have
various thicknesses. Preferred is a top of a first thickness and a skirt wit a
second thickness. The second thickness can be at least 25% larger that the
first thickness, such as at least 50%, such as at least 75%, such as at least
100%, such as at least 125%, such as at least 150%, such as at least 175%, such
as at least 200%, such as at least 225%, such as at least 250%, such as at
least 300%, such as at least 350%, such as at least 400%, such as at least
500%.
Preferred is a cup-shaped device with a second thickness
that is about 200% of the first thickness.
In an embodiment the thickness of the outer area is at least
5% larger than the thickness of middle area. The thickness of the middle area
is measured at the location where the middle area is thinnest and the thickness
of the outer area is measured at the location where the outer area is thickest.
The thicker outer area compared to the middle area provide the device with an
increased stability in the outer area, especially when compared to a device
with a uniform or substantially thickness as of the middle area. The stability
of the device implies that the device to a lesser degree bent or flex in the
outer area when located in the joint. Especially a cup-shaped or substantially
cup-shaped device has the advantages as mentioned.
In the manufacture of the device a polymeric material
optionally comprising one or more metal components as described elsewhere, has
to be spread within the device. This becomes easier when the outer area is
thicker than the middle area. Thus a reproducible production of uniform devices
or substantially uniform devices becomes more steady, hereby increasing the
quality of the devices.
A further advantage of a thicker outer area than the middle
area of the device is that it is easier to finish or close the edge of the
device. The device can be closed by a collar as described elsewhere herein.
A thin device increases the flexibility of the device,
hereby the mammal such as a human is subjected to lesser degree of
inconveniences when the device is located in a joint of this mammal. A thick
outer area thus increases the stability of the device without decreasing the
stability of the device.
In an embodiment the device further comprises at least a
second polymeric compo- nent optionally comprising a second metal component,
wherein the chain length of the first polymeric component may be longer than
the chain length of the second polymeric component.
A device according to the invention comprises at least a
first polymeric component and a second polymeric component, each optionally
comprising one or more metal components, wherein the chain length of the first
polymeric component is longer than the chain length of the second polymeric
component. The first polymeric component is providing the physical properties,
such as strength of the device as discussed below. Due to the longer chain
length the strength, in particular the tensile strength, of the device is
increased. The chain length of the first polymeric compo- nent is preferably
above 100 monomer units, such as above 120 monomer units, preferably above 150
monomer units. The chain length of the second polymer is preferably at most 99%
of the chain length of the first polymer, such as at most 95%, such as at most
90%, such as at most 80%, such as at most 70%, such as at most 60%, such as at
most 50%, such as at most 40%, such as at most 30%, such as at most 20%, such
as at most 10%, such as at most 5%.
In an embodiment the first polymeric component is selected
from polymers having a carbon-backbone.
The first polymeric component may be selected from
polyacrylates, polystyrene, polyethers, polytetrafluorethylene,
polyvinylalcohol, polyethylene, polypropylene, polyolefinic polymers,
polyethylene, polypropylene, polyethylene oxides, polyvi- nylpyrrolidon,
polysilanes, polyurethanes, polyethers, polyamides, polyesters, polyalkyl
acrylates, nylon, rubber and epoxy resins. It should be understood that the
above list of polymers is not exhaustive, and other polymers may also be
employed in the present invention. Preferred is polyethylene and polypropylene.
Most preferred is polyethylene.
When the device is constituted by two polymeric components,
each optionally comprising one or more metal components, the second polymeric
component may be selected from polyacrylates, polystyrene, polyethers,
polytetrafluorethylene, polyvinylalcohol, polyethylene, polypropylene, polyolefinic
polymers, polyethylene, polypropylene, polyethylene oxides,
polyvinylpyrrolidon, polysilanes, polyurethanes, polyethers, polyamides,
polyesters, polyalkyl acrylates, nylon, rubber and epoxy resins. Preferred
combinations for the first and the second polymeric component are polyethylene
and polypropylene, polyethylene and polyethylene, or polypropylene and
polypropylene, in the latter two cases, the first and the second polymeric
components is comprised of identical monomers, whereas the polymers thereof are
of different chain length. When the monomers of the two polymeric components
are identical the prosthetic device is preferably compounded to form a
bidispergent system.
The device is to be substantially composed of polymeric
material optionally compris- ing one or more metal components, particularly
solid or semi-solid polymers. Poly- mers are the family of synthetic or natural
macromolecules consisting of inorganic, organic polymers and combinations
thereof. Organic polymers may be natural, synthetic, copolymers, or
semisynthetic polymers. Natural polymers comprise of the class of compounds
known as polysaccharides, polypeptides, and hydrocarbons such as rubber and
polyisoprene. Synthetic polymers comprise elastomers such as nylon, polyvinyl
resin, polyvinyl chloride, polyvinyl dichloride, polyvinylpyrrolidone,
polyethylene, polystyrene, polypropylene, polyurethane, fluorocarbon resins,
acry- late resins, polyacrylates, polymethylmethacrylate, linear and
cross-linked polyethylene, phenolics, polyesters, polyethers, polypyrolidone,
polysulfone, polyterpene resin, polytetrafluoroethylene, polythiadiazole,
polyvinylalcohol, polyvinylacetal, polyvinyl oxides, and alkyds. Semisynthetic
polymers may be selected from cellu- losics such as rayon, methylcellulose,
cellulose acetate and modified starches. Polymers may be atactic,
stereospecific, stereoregular or stereoblock, linear, cross- linked, block,
graft, ladder, high, and/or syndiotactic. The term graft polymer is in- tended
to mean copolymer molecules comprising a main backbone to which side chains are
attached. The main chain may be a homopolymer or copolymer and the side chains
may contain different inorganic or organic constituents.
The device may comprises cross-linked polymers elastomers
such as high consis- tency elastomers, rubber, elastin and collagen. The
material may be selected from polyurethane, elastin, collagen and combination
products thereof. Alternative embodiments of materials suitable for the surface
of a device according to the invention include, in addition to the materials
mentioned supra and infra include hyaluronic acids and derivatives thereof.
Preferred polymeric materials are however presently believed
to be those selected from the group comprising polyolefins, such as polyethylene,
polypropylene, polybu- tene, polyisoprene, and polyvinylpyrrolidone,
combinations thereof, their copolymers, and grafted polymers thereof,
particularly polyethylene and polypropylene, most particularly polypropylene.
These polymers may be combined with metal, which is in a form described
elsewhere herein.
Preferably, the polymer materials of the first, second
and/or third polymer layer, each optionally comprising one or more metal
components, may be from the group of polyethylenes or the group of polypropylenes
such as polyethylene (PE), polypropylene (PP), high molecular weight
polypropylene (HMWPP), high molecular weight polyethylene (HMWPE), ultra high
molecular weight polyethylene (UHMWPE) and ultra high molecular weight
polypropylene (UHMWPP), high density polyethylene (HDPE), low density
polyethylene (LDPE), high density polypropylene (HDPP) and low density
polypropylene (LDPP), ultra high density polyethylene (UHDPE), ultra high
density polypropylene (UHDPP), cross-linked polyethylene, non-cross-linked
polyethylene, cross-linked polypropylene, and non-cross-linked polypropylene.
In this embodiment of the present invention, any combination of polymers listed
above, or their equivalents, may be used. These polymers may be combined with
metal, which is in a form described elsewhere herein.
In an embodiment the first, second and/or third polymeric
component is a composite material. The device may be fully or partly fabricated
of a composite material. This composite material may be a fibre-reinforced
composite material comprising fibre embedded in a polymeric matrix. After
embedding of a fibrous material in a polymeric matrix, the resultant
fibre-reinforced composite material is shaped and is partially or fully cured
to the point of sufficient hardness to provide a component for use in the
fabrication of the medical device.
The reinforcing fibre element of the fibre-reinforced
composite may be formed of one or more materials selected from the group
consisting of: graphite fibre, polyaramid, polyesters, polyamides, nylon fibre,
carbon fibre, glass fibre, collagen fibre, ceramic fibre, polyethylene fibre,
poly(ethylene terephthalate), polyglycolides, polylactides, stainless steel
fibre, cobalt-chrome alloy, titanium, titanium alloy, or nickel-titanium shape
memory alloys, biocompatible polymeric materials, and other natural and
synthetic materials compatible with the polymeric matrix.
The composite materials can also include reinforced
plastics, or polymers which are laminated or layered or reinforced with one or
more other materials such as nylon, graphite fibre, Kevlar.RTM. fibre,
stainless steel fibre, etc., nylon fibre, carbon fibre, glass fibre, collagen
fibre, ceramic fibre, polyethylene fibre, poly(ethylene terephthalate),
polyglycolides, polylactides, and combinations thereof.
The polymeric matrix element of the fiber-reinforced and
particulate-filled compos- ites is selected from those known in the art of
materials used for medical devices, including but not being limited to
polyamides, polyesters, polyolefins, polyimides, polyarylates, polyurethanes,
vinyl esters or epoxy-based materials. Other polymeric matrices include
styrenes, styrene acrylonitriles, ABS polymers, polysulfones, poly- acetals,
polycarbonates, polyphenylene sulfides, and the like. Other polymeric mate-
rials mentioned herein can also be used.
The reinforcing material may be in the form of a network of
fibre formed of one or more materials as mentioned above, where the network is
embedded in one or more polymers. The network or the fibres are embedded in a
polymer, where the poly- mers are described elsewhere herein.
In the method of the present invention, the device is made
after the embedding of the fibre with a polymeric matrix. After embedding of
the fibre, the resultant composite material is formed into, for example, a long
bar and cured or polymerized to a hardness whereby the bar may be cut and/or
machined without deforming the structural integrity of the bar. The bar is
preferably cut into short segments and is ready for use in the fabrication of
medical devices. The bars may be used as they are or may be further modified by
cutting, grinding, machining, heating and shaped and the like to provide a
specifically shaped or customized component.
The medical device in accordance with the present invention
is preferably formed from a fiber-reinforced composite material comprising a
polymeric matrix and reinforcing fibre within the matrix. The fibre are
embedded in the matrix manually or mechanically by a variety of techniques
including, but not limited to matched die proc- esses, autoclave moulding,
resin injection moulding (RIM), sheet, dough and bulk moulding, press moulding,
injection moulding, reaction injection moulding, resin transfer moulding (RTM),
compression moulding, open moulding, extrusion, pultru- sion and filament
winding.
It is further contemplated that the fibre or wires of metal
can be interwoven with non- resorbable polymers such as nylon fibre, carbon
fibre and polyethylene fibre, among others, to form a metal-polymer composite
weave. Further examples of suitable non- resorbable materials include DACRON
and GORE-TEX. The fibre may further be treated, for example, chemically or
mechanically etched and/or silanized, to enhance the bond between the fibre and
the polymeric matrix. The fibre preferably take the form of long, continuous
filaments, although the filaments may be as short as 0.1 to 4 millimeters.
Shorter fibre of uniform or random length might also be employed. The fibrous
element may take the form of a fabric. Fabric may be of the woven or non-woven
type and is preferably preembedded with a polymeric material as set forth
herein. The fibrous component may be present in the fiber reinforced composite
material in the range from about 20% to about 85%, and more preferably between
about 30% to about 65% by weight.
In an embodiment the first and/or second and/or third
polymeric component is nano- fibre and/or a nano-structured composite. The
nanofibre is nanofibre of any of the polymers mentioned elsewhere herein.
The composite polymeric material may be a nano-structured
composites in the form of reinforced polymers with low quantities (<25% eg.
less than 15% or less than 5%) of nano-metric sized clay particles or
nano-metric sized particles of any other material mentioned herein.
In an embodiment the first and/or second and/or third
polymeric component is cross- linked. The polymer is selected from any of the
polymers mentioned herein. The combination of a polymer having a high chain
length and a polymer having a shorter chain length, but being cross-linked
provides a strong device yet having the resilient properties necessary for the
device.
In an embodiment the first and the second polymeric
component comprises the same monomeric component or the same components of the
composite material. Optionally these polymeric components further comprise one
or more metal components, which may be of different length in the two polymeric
component.
The device and units may be designed not to interfere and to
be non-invasive with regards to intra-articular components when the device is
in the joint cavity by means such as a slit in the body of the device.
Moreover, non-interference of the intra-articular components may be achieved by
a hole which runs through the body of the device; that is to say the device may
comprise a hole through which intra-articular components may pass. When loading
the device, the slits may serve to pass intra-articular components through the
body of the device. The slits in this embodiment run from the periphery of the
body of the device to the hole through which the intra-articular components
pass after the device is implanted or loaded.
Typically, and to at least some extent, the device is
adapted in its structure and/or material composition to alleviate conditions
associated with worn cartilage by providing a spacer function and/or to exert
pressure distribution in the joint when the joint is loaded and/or to provide
at least part of the sliding/rotating movement of the joint by internal
movement of at least part of the device.
It is also an object of the present invention to provide a
method for non-invasive locking of a device within a joint. In addition, the
method may be independent of use of cement or bony ingrowth of the device.
A still further object of the present invention is to
provide a kit for use in the method for non-invasive locking of a device within
a joint.
It is also an object of the present invention to provide a
method for preventing damage between mating surfaces or articulating surfaces
within a joint such as between the femoral head and the acetabulum of a hip
joint.
A more specific object of the present invention relates to a
prosthetic device for insertion into a joint cavity of a joint of a vertebrate
such as a human, the device is being adapted to provide a spacer function
and/or to exert stress distribution in the joint when the joint is loaded
and/or to provide at least part of the sliding/rotating movement of the joint
by internal movement in the material of at least part of the device, the device
being capable of being fixed or retained in the joint cavity in a manner which
is substantially non-invasive with respect to cartilage and bone natively
present in the joint cavity. When inserted into the joint cavity the device can
be constructed to locking itself to an intra-articular component and thereby
being fixed or retained in the joint cavity.
Physical-Structural
Features of the Device
The physical-structural features of the device relate to the
size, form or shape of the device as well as the structural components and
design components of the device.
Size and Shape
The overall shape of the device is such that it
substantially fits into the excising anatomical dimensions of the joint. In
general, the size and shape of the device may be such that the device fits into
the intra-articular cavity in that it may partially or fully occupy the space
defined by the cavity, this depends on the type of device. For some of the
joints it is preferred that the extent of the device, when positioned in the
joint cavity, is larger than the normal extent of cartilage on the bone end in
that joint. In an embodiment, a hole runs through the body of the device to
allow intra-articular components to traverse the body of the device and thus be
surrounded by the device.
In this embodiment, the device may be construed in a liberal
sense as essentially torus-shaped in that the device can be of a plurality of
geometrical shapes, symmetrical and asymmetrical, comprising a hole which runs
through the body to create an internal tubular passage through which
intra-articular components may pass.
The device may also be ball-shaped, disc-shaped, spherical,
globular-shaped, cup- shaped, cone-shaped, ring-shaped, cylindrical and have
convex, concave, or flat surfaces. Accordingly, the body of the device shape
can e.g. be in the form of a horseshoe, a curl, ring-shaped, circular or
semicircular so as to be suitable for fitting into the anatomical dimensions of
the particular joint. Furthermore, the device may be asymmetrical.
The body of the device may be of a geometrical shape
comprising a surface having the form of body shaped by rotating a circle about
a coplanar axis which does not intersect the circle. It may be ball-shaped,
disc-shaped, globular-shaped, cup- shaped, cone-shaped, ring-shaped,
cylindrical and may comprise convex, concave, or flat surfaces. In some aspects
it is characterised in that it comprises a hole extending from one surface of
the body of the device to the same or another surface, creating an internal
tubular cylinder. This internal tubular cylinder may be straight if the hole
extended to two parallel surfaces, curved if the hole extends to perpendicular
surface, U-shaped if the hole extends to two parts of the same surface or a
combination of one or more of these internal shapes and thus tortuous.
Certainly, given that the overall shape of the device is
such that it substantially fits into the excising anatomical dimensions of the
joint, it is anticipated that the body of the device may be asymmetrical or of
no definable shape so as e.g. to the fill the intra-articular cavity, to allow
for the movement of the intra-articular components during the flexing of the
joint, to support intra-articular components or to support matter which form
the walls of the cavity.
It is preferable that the shape of the device is such that
it does not impede the normal functioning of the joint and its components.
It is particularly anticipated that the body of the device
may be asymmetrical or of no definable or uniform shape when the device is for
use in a hip joint. Alternatively, the shape of the device may be such that it
resembles the native cartilage, or part thereof, naturally present in the joint
cavity.
Accordingly,
in the case of a hip joint, the shape of the device is preferably such that it
fits into the existing space of the joint cavity comprising ligamentum capitis
femoris, the "walls" of the space being defined by the concave shape
of the acetabulum and by the convex shape of the femoral head.
Moreover, the overall shape of the device may be a result of
an assembly of more than one units of the device, such as the assembly of two
or more rings of different sizes stacked upon each other so as to form a
cone-shaped device. The assembly of units may be done in vivo or ex-vivo.
Furthermore, in an embodiment, the overall shape is such
that the device is capable of locking itself to an intra-articular component if
present in the joint and thereby being fixed or retained in the joint cavity.
When the intra-articular component is a ligament, the shape is such that the
ligament is surrounded or substantially surrounded by the device.
However, the overall shape of the device may have any other
form as long as the material is of such a character that the device when
present in situ fits into the joint cavity, for example due to elastical
deformation of the device.
Preferably, the elastical deformation of the device is such
that the presence of liga- mentum capitis femoris results in a shape leaving
room for the ligamentum. Otherwise, the surface of the upper part of the device
facing the acetabular cavity may comprise a groove embedding the ligament.
Typically, the shape of the device is formed from a moulding
of its materials or from a casting process. It may alternatively be the result
of a framed structural construction or skeletal assembly. It is typically solid
in that the body of the device is not hollow but rather such that the material
of the device comprises all or essentially all of the space between two
surfaces. The moulding, casting, construction or assembly may form a device
into a uniform or non-uniform shape.
The device is essentially uniform in its stiffness or
compressibility. However, when loaded, the material may have a tendency to
deform in such a way that the locking mechanism is altered. This may occur if
the element adapted to surround the ligament, when present in situ, has a slit
which expands or gapes upon loading when the device is pressed together. This
gaping may be further pronounced when the patient is e.g. walking whereby the
ceiling of the acetabulum is pressed down on the upper surface of the device
and the lower surface of the device is pressed down on the spherical surface of
the femoral head.
Due to the rolling movement (rotation within the joint) of
the femoral head, the possibility exists that the femoral head may press itself
up into the slit of the device during the movement. In such cases, the press
distribution and/or internal movement of the device may be limited to a minor
part of the device that may result in an undesirable increased pressure on that
portion of the device. Finally, contact between the femoral head and the
acetabulum may occur in case the femoral head penetrates through the device.
However, a device comprising parts overlapping each other can prevent this
possible undesirable effect.
Accordingly, as mentioned above, the device may be
curl-shaped whereby the de- vice with respect to the slit or opening has
overlapping parts which do not represent a complete opening in the loading
direction.
The size of the prosthetic device according to the invention
may be of any size corresponding to the dimensions of the joint. In a hip
joint, a suitable size is normally one that allows the diameter of the device
to be about the same or less than the diameter of the femoral head. However, on
some occasions the diameter may exceed that of the femoral head. The size may
also depend on the degree of damage of the native cartilage of the joint.
Moreover, the space available within the joint in the individual may have an
effect on the preferred diameter. Also the compressibility of the material
should be taken into account. In the case in which the material is highly
compressible, the device may increase in diameter upon loading of the joint;
when loaded, the device should generally cover the surface area which is
covered with cartilage in the normal joint, e.g., in the hip joint, the surface
of caput femoris should preferably be substantially covered when the joint is
loaded to avoid contact of the surface of the femoral head with the acetabulum.
The length of the diameter of the device is designed to fit
into the particular joint, such as between 1-80 mm, such as between 2-70 mm,
preferable between 10-60 mm, more preferable between 15-50 mm, most preferred
about 40 mm, when the joint is loaded.
The prosthetic device according to the invention may vary in
thickness depending on the load on the joint, and the thickness of the device
may also vary within the device.
The thickness of the device in each of the middle area and
outer area is at least 0.1 mm, preferably between 0.2-60 mm, such as between
0.3-40 mm, preferably 0.6-30 mm, more preferably about 0.8-20 mm, most
preferably about 1-15 mm in the unloaded stage. Depending on the material, the
device may be highly compressible, whereby the initial thickness may exceed the
above-mentioned upper limit. If only a limited rotation takes place in the
joint, the thickness of the device may be decreased.
In one embodiment of the invention, the device is capable of
locking itself to the in- tra-articular component by at least one element of
the device surrounding the component in such a manner that displacement of the
element, and thereby the device, is limited by interlocking with the component.
The intra-articular component which is surrounded is preferably a ligament,
such as a ligament natively existing in the joint cavity.
In one embodiment of the device according to the invention,
the element completely or substantially completely surrounds the ligament.
Thus, one embodiment relates to a prosthetic device
according to the invention re- lates to a device wherein the element
interlocking with a ligament, when present in situ, permits the ligament to
extend through the element and substantially exert its natural function on the
joint.
In one aspect of the invention, the prosthetic device is
intended for the articulation of a hip of a human, the device being adapted
such that when present in situ in the human hip joint cavity, it comprises at
least one element surrounding ligamentum capitis femoris. Accordingly,
ligamentum capitis femoris represents the surrounded intra-articular element
mentioned above.
It is contemplated that the surrounding of the intra-articular
component by the element may be a completely or substantially completely
encircling of the ligament.
It is also preferred that the prosthetic device, when
present in situ, comprises at least one ring-shaped or substantially
ring-shaped element.
According to another aspect of the invention, the element of
the prosthetic device which is adapted to surround the ligament when present in
situ has such a shape and such properties that it can be placed around the
ligament and stay interlocked with the ligament.
Structural Components
The device preferably comprises structural components which
permit arrangement of the body of the device around native intra-articular
components.
When the prosthetic device according to the invention is a
hip endoprothesis, the device has a shape and structural components permitting
arrangement of the body of the device around ligamentum capitis femoris.
A prosthetic device according to the invention comprises a
device wherein the element of the device interlocking with the device with an
intra-articular component has such a shape and/or properties that it is capable
of replacing or supplementing worn or damaged cartilage in the joint and/or is
capable of preventing wear of the native cartilage of the joint or of the bone
tissue of the joint.
The structure of the material of the device or of a part of
the device may be in the form of fibres and filaments of polymers and/or metal
which can be incorporated into the matrix in a braided, woven, spongy or spiral
pattern, the fibres and filaments having reinforcing properties. The polymer
fibres may be inorganic fibres such as carbide, nitride, boride, carbon and
oxide fibres, or the polymeric reinforcement may be of organic origin such as
Dacron™. In a preferred embodiment the fibres are selected from polyethylene
fibres, polypropylene fibres or a combination thereof. The metal may be any
suitable metal e.g. titanium, gold, silver and/or chromium- cobaltum, and may
be of any structure as described elsewhere herein.
The structure of the material of the device may comprise a
layered or laminated structure, a core of one material or one or more
interposed layers with different properties enabling an overall function of the
devise suitable for providing a spacer function and/or to exert pressure
distribution in the joint when the joint is loaded and/or to provide at least
part of the sliding/rotating movement of the joint by internal movement of the
device, or relevant part of the device. However, it is preferred that the
material itself does not comprise interposed layers resulting in sliding
between the layers and thereby tear on the mating surfaces within the device.
Accordingly, the body of the device should be one continuous solid or
semi-solid material. In one preferred embodiment of the invention, the device
comprises a tubular passage through which the ligament can pass and be
surrounded by the body of the device. Circular movement around the
substantially central ligament is possible but replacement of the device is
prevented. A further feature of the structure of the de- vice may be that of a
slit extending from the outer surface of the device and through the body of the
device into the central tubular passage. The slit may be curl-shaped in the
radial direction with the axis of the tubular passage being the centre.
The slit may curl or curve into the body of the device so as
to form an S-, or C- shaped slit, or zigzag or spiral slit. The curl of the
slit may be in the two dimensions of a disc shaped device, or may curl in all
three dimensions in the case of a globular, spherical, cone-shaped or
cup-shaped device.
Furthermore,
in embodiments where the device comprises more than one unit, the curvature of
the slit may be such as to form a zigzag, spiral or S- or C-shaped multi- unit
slit.
In multi-unit devices, the outer surfaces of the parts of
the unit which are in contact with each other may have a surface pattern
preventing the units from sliding apart such as grooves or etching or jagged
surface pattern.
Moreover, the overall shape of the device may be from an
assembly of two or more elements of one device, such as two semi-circular
elements assembled to form a ring or from the assembly of two elements
obtainable from the cross-sectioning of a ring or globular device along their
longest axis. As was the case for the surface of two units, two elements may
have a surface pattern preventing the elements from sliding apart such as
grooves or etching or jagged surface pattern. Thus, a device and its shape may
be the result of an assembly of two or more elements and/or two or more units,
each comprising surfaces designed to preventing slippage of units and/or
elements.
If suitable, the device may comprise a material which
functions as a frame for the shape or secures the device from opening when
placed in situ, for example in the form of a shaped component having the
properties of a spring or the like. In one embodiment, the ring-shaped body of
the device has a slit or other suitable means which enables the device to be
placed in the position encircling ligamentum capitis femoris.
Upon loading the device into the joint, the element of the
device surrounding the component, e.g. a ligament, and thereby interlocking
with the component, may tend to open up due to deformation of the device in the
form of flattening resulting in an increased diameter. When the diameter of the
device increases, e.g. the diameter of a ring-shaped device comprising a slit,
the adjoining surfaces of the slit may gape.
As stated, during the compression, extension or rotation of
the device when the device is present in a joint, the slit may have a tendency
to gape and thus result in reduced weight-bearing effectiveness and/or result
in trapping of intra-articular components within the seam of the slit.
Preferably, the seam cannot be pulled apart in the direction of the plane of
the seam by the mechanical pressure exerted by the body of the device conferred
by the elastic properties of the material.
To prevent undesired slippage of the seam perpendicular to
the plane of the seam, a variety of means may be incorporated into the design
of the device so as to lock or adhere the two sides of the seam. Preferably,
the locking or adherence means are reversible so as to allow removal or
manipulation of the device after initial loading and use.
The seam is preferably characterised in that a smooth
surface is formed in the plane of the seam.
To prevent the device from opening, the device preferably
comprises overlapping or intersecting parts, such as lips or dovetails as is
known by the person skilled in the art of mechanics or moulding. The two sides
of the seam may be adjoined by means of an interlocking device such as a
protrusion-hole device on sides of the seam. Alternatively, to prevent slippage
in perpendicular to the plane of the seam, each side of the seam may be such
that each side of the seam comprises an alternating sequence of angled grooves
and corresponding extrusions. Moreover, the top and bottom portion of each side
of the seam may comprise alternating teeth and sockets to prevent slippage. To
prevent gaping such overlapping parts and their mating sur- faces of the sides
of the seam may have an interlocking surface structure. The pattern of such a
structure may include depressions on the mating surface of one part and corresponding
elevations on the other mating part of the device.
Accordingly, in one embodiment, the overlapping parts are
such that the interlocking surface structures constitute grooves. These grooves
may extend radially, primarily resulting in a decreased tendency of the device
to "open up" at the area corresponding to the slit or the gap. The
grooves may also be orientated in a circulatory structure preventing the mating
surfaces from gliding or sliding apart from each other. Additionally, the structure
may comprise a combination of both elements reducing undesired movement in both
of the two directions, when the device is deformed during loading of the joint.
The terms "radially" and "circular"
should be understood as relative to the centre of the device or relative to the
part of the device where the ligament extends through the device.
"Radially" meaning e.g. grooves being located along radii from the
centre, and the term "circular" meaning that e.g. the grooves are
located along the periphery of a circle around the centre.
In another embodiment, the pattern includes other
prominences or knobs, including pointed elevations. Thus, any structure
comprising an elevation on one mating surface and a corresponding depression on
the other mating surface may result in a decreased movement between the mating
surfaces. Accordingly, any structure of the mating surfaces which thereby
functions as an interlocking "hook" is within the scope of the
invention. The mating surfaces of the curls may have an interacting profile in
the form of a shape or pattern such as grooved surfaces which prevent the
surfaces from sliding apart by reducing sliding movements between the mating
surfaces upon loading of the device.
Another preferred embodiment of the invention relates to the
seam created by the slit in the body of the device, accounts for preventing of
slippage or gaping of the seam by means of a chemically treated surface of the
sides of the slit. One embodiment of this aspect of the invention anticipates
adherence of the two sides of the seam by means of photolytically or thermally
activating a reaction between the chemically treated surfaces of the sides of
the seam once the device has been loaded into the joint. Preferably, this
adherence is reversible.
In another embodiment, the device may also comprise two or
more separate rings each having a slit which are arranged so that the slits are
orientated in such a way that no direct opening exists in the loading
direction, accordingly, the slits are displaced in the direction parallel with
the axis of the device. Mating surfaces of such rings may also have an
interlocking structure as explained above.
In a still further embodiment, the device is in the form of
a curl, wherein the ring- shaped elements together have the overall shape of a
cup. Also in this embodiment, the mating surfaces may comprise grooves
preventing sliding movements of the mating surfaces upon loading.
In a still further embodiment, the device may comprise minor
vertical slits on the outer periphery of the device, these minor slits, e.g.,
having a depth of 1-5 mm may "absorb" the increasing diameter of the
device upon loading. Preferably, the part of the device comprising the slits
(the outer periphery) is not subject to heavy loading which could result in
particulation of the edges of the device corresponding to the slits. These
minor vertical slits on the outer periphery of the device may alternatively serve
so as to not interfere with movable or immobile components of the joint within
the cavity.
The device according to the invention may e.g. be processed
by moulding of the material including extrusion and injection moulding.
However, any other means for preparing the device of the desired shape could be
utilised.
In addition, the device may comprise a dye or other material
enabling visualisation of the device such as by X-ray.
Material Features
The material features of the device related to features conferred
by the chemical composition of the device. It is well known in the orthopaedic
field to use different types of materials for prostheses that are suitable for
implantation in the body. The device may be produced from any material or
combination of materials suited for implants. However, it is preferable that
the body of the device does not comprise of any substantial extent of metallic
materials.
The combination of materials can be varied according to the
properties preferred for each device. However, for some implant types, the body
of the device is constituted of polymeric material or materials optionally
comprising one or more metal compo- nents.
Preferably, the material of which the device is made is
biocompatible, e.g. hemo- compatible, thromboresistant, non-toxic, and/or
non-carcinogenic. In addition, the material should be resistant to
particulation, and the solid surface of the material should be so that the
surface tension is suitable for the interaction between the material and the
biological surfaces.
Biocompatibility may be assayed through in vitro tests as
well as animal tests. Enzymatic biodegradation may be used as indicative of
biocompatibility. Furthermore, chondrocytes and fibreblasts may be grown on the
material to evaluate the compatibility.
Finally, biocompatibility may be evaluated by implanting
devices of the material in animals and examining the animal and/or device after
a period of time.
Polymers and copolymers of polypropylene or polyethylene, as
well as grafted forms of each of these are particularly interesting. Moreover,
surface treated forms of these polymers, copolymers or grafted polymers are of
notable interest.
The structure of the polymeric material of the device or of
a part of the device may be in the form of fibres and filaments which can be
incorporated into the matrix in a braided, woven, spongy or spiral pattern, the
fibres and filaments having reinforcing properties. The fibres may be inorganic fibres such as
carbide, nitride, boride, carbon and oxide fibres, or the reinforcement may be
of organic origin such as Dacron™. In a preferred embodiment the fibres
are selected from polyethylene fibres, polypropylene fibres or a combination
thereof. The fibres may be surface treated before incorporated into the matrix
to obtain a better adhesion of fibres to matrix.
The present invention in particular relates to a device
composed of material formula- tions intended to meet the specifications of
durability, biocompatibility, etc. These properties are obtainable by treating polymer
materials, such as polyethylene, polypropylene or polyvinylpyrrolidone or
combinations and co-polymers thereof as well as precursor materials for
polymerisation, with high-energy electrons, gamma rays, photons, microwaves,
ion implantation, plasma treatment, annealing, thermal radia- tion or another
radiation to obtain ideal durability and biocompatibility of the new, modified
material. Treatment of the above-mentioned materials with radiation leads to
cross-linking of polymers and thereby generating new, modified materials.
Preferably, the polymer material is a cross-linked polypropylene material. In
another embodiment the polymer material is a cross-linked polyethylene
material.
In one embodiment the device comprises a body constituted by
the first and the second polymeric components, each polymeric component
optionally comprising one or more metal components. The body may optionally be
treated in order to optimise the properties such as surface properties,
biocompatibility and/or low friction. By the term "body of the
device" is meant the part of the device providing the strength properties
as well as the resiliency properties.
In another embodiment the device comprises a body
constituted by the first polymeric component, whereas the second polymeric
component provides optimised surface properties.
Furthermore, radiation also allows grafting of polymers onto
existing polymer surfaces, resulting in new mechanical properties as well as
new surface properties. In this manner, the resulting modified polymer device
can be processed to meet the necessary requirements of durability and
biocompatibility.
Polymers may be prepared by methods known to the person
skilled in the art. Chemical catalysis, thermal induction or photo induction
are anecdotal non-limiting examples of methods of preparing the polymers. The
cross-linking of the polymers or grafting may be done by radiation or other
methods known to the person skilled in the art.
The properties of the materials to be obtained by these
cross-linking and grafting processes are preferably i) resistance to tear and
wear; ii) good compressibility; iii) flexibility and surface properties which
will allow wetting with biological fluids, and/or eventually allow growth of
chondritic cells onto the prosthetic device.
Typically, the device is prepared by a process comprising of
the following steps:
• The prosthetic devise may be formed by casting the pure
polymer optionally comprising one or more metal components, or a blend of
polymers optionally comprising one or more metal components in a mould of
specified dimensions. The polymer is chosen from the above mentioned polymers.
The metal compo- nent may be chosen from the metals mentioned elsewhere herein.
• After hardening the cast material as formed, or after
swelling in a suitable solvent, the device is subjected to high-energy
electrons, gamma rays or another radiation in order to create cross-linking
which will modify the mechanical properties of the cast material to meet the
preferred specifications.
• Finally,
eventually after removal of the swelling solvent, the surface of the cast
material is treated to achieve good surface properties as described above.
The surface of the device can subsequently be treated to
modify surface properties such as wetting ability and/or biocompatibility. This
surface treatment can be per- formed by plasma treatment, chemical grafting or
by a combination of plasma treatment and chemical grafting. The surface of the
device contacting with the articulating surfaces of the joint may be of such a
material which forms a uniform contact surface reducing the overall contact
stress per unit area, and thereby avoiding corrosion of the articulating
surfaces of the joint. Accordingly, the material contacting with the biological
surfaces may be smooth, biocompatible, preferably self- lubricating, and it
should be wear-resistant so that powder generated due to wear is avoided in
that this could otherwise result in foreign matter reactions and cause further
trouble to the function of the joint. Furthermore, the surface material should
preferably be a material or a combination of materials having self-repairing
properties so that fissures, cracks or other ruptures on the surface do not
exceed uncontrollable levels. However, the surface material is preferably
continuous with the material of the rest of the device, e.g. the material may
gradually merge into the material of the inner core or matrix of the device.
The surface of the material may be chemically treated so as
to soften, rigidify or lubricate the surface of the device or parts thereof.
The surface of the material may be coated so that the coating confers these
properties, or may be treated so as to chemically alter the surface of the
device so as to confer any of these properties. Alternatively, certain polymer
surfaces may be modified by means of thermal or photolytic energy.
Also the surface treatment may be provided by incorporating
surface treatment polymer, such as polyvinyl pyrrolidone, into the matrix to
maintain the good surface properties.
Independent of whether the body of the device comprises one
or two components, it is preferred that the body of the device is provided with
a treatment resulting in a functional surface of the device being wettable by
the joint fluid normally present in the joint cavity, in oder to decrease any
friction between the device and joint parts, such as bone, cartilage, ligaments
and mucosa.
Without being bound by theory it is also believed that a
wetted surface reduces the risk of having the immune system recognising the
device when implanted, which would otherwise lead to adverse effects of the
device.
By the term "functional surface" is meant the
external surface of the device, ie. the surface contacting joint cavity parts.
Since the body of the device is often produced as one, two or even three
dimensional networks, internal surface may be present in the body, the internal
surfaces often corresponding with the external surfaces.
The prostheic device may alsocomprises a third polymeric
component, the third polymeric component being different from the first and/or
the second polymeric component. The third component will preferably be grafted
to the body of the device and result in the improved surface properties. The
third polymeric component is preferably selected from polyethylene oxides, and
polyvinylpyrrolidon, most preferably from polyvinylpyrrolidon.
When the body is comprised of one component, such as wherein
the first polymeric component comprises a copolymer of polyethylene and
polypropylene or wherein the first polymeric component is a cross-linked
polymer, the second polymer may be grafted to the first polymer and act as the
third polymeric component as described above. The first and/or second polymeric
component may optionally comprises one or more metal components.
Preferred devices are composed of:
A body of polyethylene having polyvinylpyrrolidone grafted
thereto A body of two polyethylene polymers of different chain lengths having
polyvinylpyrrolidone grafted thereto
A body of polypropylene having polyvinylpyrrolidone grafted
thereto A body of two polypropylene polymers of different chain lengths having
polyvinylpyrrolidone grafted thereto A body of a copolymer of polyethylene and
propylene having polyvinylpyrrolidone grafted thereto
A body of a polyethylene and a copolymer of polyethylene and
polypropylene having polyvinylpyrrolidone grafted thereto A body of
polypropylene and a copolymer of polypropylene and polyethylene having
polyvinylpyrrolidone grafted thereto
A body of polyethylene having 2-vinylpyrrolidone grafted
thereto
A body of two polyethylene polymers of different chain
lengths having 2- vinylpyrrolidone grafted thereto
A body of polypropylene having 2-vinylpyrrolidone grafted
thereto A body of two polypropylene polymers of different chain lengths having
2- vinylpyrrolidone grafted thereto
A body of a copolymer of polyethylene and propylene having
2-vinylpyrrolidone grafted thereto A body of a polyethylene and a copolymer of
polyethylene and polypropylene having 2-vinylpyrrolidone grafted thereto A body
of polypropylene and a copolymer of polypropylene and polyethylene having
2-vinylpyrrolidone grafted thereto
In the list polyvinylpyrrolidone and 2- vinylpyrrolidone
need not be grafted to the de- vice.
Insertion
It is also an object of the present invention to provide a
method for introducing a device according to the present invention into a
joint. The method comprises:
a) locking the device to an intra-articular component and
thereby fixing or retaining the device in the joint cavity in a manner which is
substantially non-invasive with respect to cartilage and bone natively present
in the joint cavity.
The method may further comprise any of the following steps
before locking the device to the intra-articular component in the joint:
i) exposing the joint capsule by conventional surgery procedures,
ii) penetrating the joint capsule into the joint space
leaving a passage for
iii) introducing the prosthesis into the joint capsule via
the passage, the prosthesis having a shape suitable for being introduced
through this passage.
Locking the device to the intra-articular component and
thereby fixing or retaining the device in the joint cavity in a manner which is
substantially non-invasive with respect to cartilage and bone natively present
in the joint cavity may include encircling a ligament present in the joint with
a ring-shaped element of the device such as a ring-shaped device having a slit
extending from the periphery of the device to the central opening of the
"ring".
The method may further comprise the steps of deforming the
prosthetic device into a reduced volume or a slender shape before locking the
device to the intra-articular component. In the case of insertion into a hip
joint, the insertion of the device is preferably performed after penetration
through the head of the rectus femoris muscle leaving a passage having a
substantial width for introducing means into the joint capsule without
alteration of the function of the capsule after the surgery.
Means or instruments for inserting the device into the joint
space can be in the form of forceps comprising means for deforming the device
into a minor volume or a more slender shape and may comprise means for grasping
around the intra-articular component to which the device is capable of
interlocking.
The forceps may further comprise means for locking the
device around or substantially around the intra-articular component and
optionally means enabling the forceps to be withdrawn without withdrawing the
device.
Thus, a
further object of the invention relates to a kit comprising:
a) an intra-articular prosthetic device for a joint having
a.1) a spacer function and/or capability to exert pressure
distribution and/or sliding/rotating movement of the joint by an internal
movement of the device by means of a resilient member, and
a.2) a locking mechanism adapted to fix the device to an
intra-articular component by means of an element of the device surrounding the
component in such a manner that displacement of the device is limited by
interlocking with the component; and
b) an instrument for inserting the prosthetic device into a
joint cavity.
Preferably, the elements of the kit should be sterile.
The instrument b) may further comprise one or more of the
following means b.1 to b.4: b.1 ) means for deforming the prosthetic device
into a reduced volume or to a slender shape and keeping this volume or shape
upon introduction of the device to the joint;
b.2.) means for grasping or encircling the intra-articular
component to which the element of the prosthetic device is capable of
inter-locking;
b.3.) means for leaving the prosthetic device in the joint
with the element of the prosthetic device surrounding an intra-articular
component; and
b.4.) means for retracting the instrument from the joint.
It is contemplated that each of the means of b.1.), b.2.),
b.3.) and b.4.) may be con- nected to or form part of a handle. Moreover, the
resilient member of a.1) and the element surrounding the intra-articular
component of a.2) may constitute the prosthetic device.
The means of b.2.) for grasping or encircling the
intra-articular component may comprise an incision of the instrument which, in
situ, is able to substantially retain the element within the "legs"
of the incision.
Biological activity of the device
When inserted in the joint cavity the device is capable of
alleviating the pain and other symptoms related to damaged cartilage, such as
improving movements. Furthermore, the device may be capable of healing the sick
bone's structure and/or cartilage structure- in hole of partly.
For example the device may facilitate creation of new
cartilage and/or minimise de- struction, such as fibrillation and/or
fragmentation, of cartilage by relieving the pressure on the residual
cartilage/bone in the joint
Furthermore, the device may comprise biological active additives.
Medication or biological active substances can be used as additive to the
device to facilitate heal- ing, minimise destruction or with other therapeutic
goals, such as pain relieve, anti- inflammation, oncology treatments,
stimulation of bone growth, and/or anti-infectious agents. Also, biological
osteogenic or chondrogenic, chondral inductive, and/or chondral conductive
materials may be added to the device. In particular patients suffering from
osteoporosis or other bone degenerating conditions may benefit from having
devices comprising osteogenic inductive materials implanted.
The device itself can be used as a growth medium and/or
network for the natural or artificial cells, such as chondrocytes.
The device is capable of being formed in the production
process to suit any joint cavity of animals or human beings, therefore the
device may for example be formed to fit into any one of the following joints:
Hip joint, knee joint, ankle joints, shoulder joint, elbow joints, wrist,
fingers, spinal column joints, such as for substituting in- tervertebral discs,
and the jaw joint.
The material for the medical devices is primary polymers,
with at least one layer of a first polymeric component with high molecular
weight, and at least a layer of a second polymeric component with low molecular
weight. This combination of longer and shorter polymers provides the feature of
the device comprising strength as measured by tear, tension and compression.
These polymers may optionally comprise one or more metal components which is
described elsewhere.
In one of the invention a medical device comprises a
bio-compatible polymeric product with a layered structure comprising at least
one upper layer of a first polymeric component, a middle layer of a second
polymeric component, and at least one lower layer of a third polymeric
component, wherein the chain length of the first polymeric component and the
third polymeric component is longer than the chain length of the second
polymeric component. These polymers may optionally comprise one or more metal
components which is described elsewhere.
An important step of the present invention is the selection
of the composition of the different polymer layers optionally comprising one or
more metal components, as well as the selection of the number of polymer layers
comprising first, second and third polymeric components, as well as thickness
of the polymer layers and also size and position of an optional layer of the
second polymeric component. These polymers may optionally comprise one or more
metal components which is described elsewhere.
Another embodiment of the present invention provides for
layers of polyolefinic polymers and resins. Within the context of the present
invention, a polymer is defined as an organic compound having repeating units
of similar or different monomers. A resin is defined herein as a partially
cured polymer having utility as a mouldable material suitable for curing into a
solid article.
First polymeric and third polymeric component
The polymers comprising the first polymeric component and
the third polymeric component may be above 100 monomer units, such as above 1
,000 monomers units, for example above 10,000 monomer units, preferable above
20,000 monomer units, more preferable above 30,000 monomer units, further
preferable above 40,000 monomer units, yet further preferable above 50,000
monomer units, most preferable above 60,000 monomer units.
The polymers of the upper and lower layer comprising first
and third polymeric components of the present invention have preferably
molecular weights ranging between 1,000 and 100,000,000 such as between 10,000
and 75,000,000, for example between 50,000 and 50,000,000, preferable between
75,000 and 25,000,000, more preferable between 100,000 and 1 ,000,000, further
preferable between 200,000 and 800,000, yet further preferable between 300,000
and 700,000 most preferable between 400,000 and 600,000
In a preferred embodiment the polymers comprising the first
polymeric component and the third polymeric component are comprises long
polymer fibres, filaments or strands produced from the polymers presented
above, preferred polymers to produce the fibre and filaments may be selected
from the group of poly-ethylenes including, but not limited to, high molecular
weight polyethylene (HMWPE), ultra high molecular weight polyethylene (UHMWPE),
high density polyethylene (HDPE), ultra high density polyethylene (UHDPE),
cross-linked polyethylene and non-cross- linked polyethylene. The most
preferred polymer of the invention is fibre produced from UHMWPE. The polymers
of the first polymeric component and the third polymeric product provide
strength and wear resistance to the device.
A preferred polymer of the upper and lower layer of the
invention is UHMWPE, and a preferred combination is UHMWPE and HDPE.
Additional components of the upper and lower layer polymeric
material may be incorporated into the matrix in a braided, woven, spongy or
spiral pattern, the fibres and filaments comprising the additional components
having reinforcing properties. The fibres may be inorganic fibres such as
carbide, nitride, boride, carbon and oxide fibres, or the reinforcement may be
of organic origin such as Dacron.
The first and third polymeric component may also be any
other polymeric component described elsewhere herein.
The second polymeric component
The middle layer comprising a second polymeric component can
be constructed from short chain polymer material; the polymers may be selected
from the polymers presented above. Short chain polymers may have less than
about 100 units, such as less than about 90 units, for example less than about
80 units, preferable less than about 70 units, more preferable less than about
60 units, further preferable less than about 50 units, yet further preferable
less than about 40 units, most preferable less than about 30 units. The short
chain polymer material may not have cross links and only weak Van der Waals
forces between chains, The molecular weight is preferably less than about
10,000, such as less than about 9,000, for example less than about 8,000,
preferable less than about 7,000, more preferable less than about 6,000,
further preferable less than about 5,000, yet further preferable less than
about 4,000, most preferable less than about 3,000.
Preferred polymers to produce the middle layer constituting
film, core and inlay polymer layers may be selected from the group of
poly-ethylenes or from the group of polypropylenes including, but not limited
to polyethylene (PE), polypropylene (PP), high molecular weight polyethylene (HMWPE),
high molecular weight polypropylene (HMWPP), high density polyethylene (HDPE),
high density polypropylene (HDPP), low density polyethylene (LDPE) and low
density polypropylene (LDPP). Preferred is short chain polymer material such as
LDPE and LDPP. Further preferred are polymers which are branched. Most
preferred is short chain polymer material of polyethylene.
From the above mentioned first, second and third polymeric
components different polymer layers comprising fabric, film, core and inlay are
constructed. These polymer layers are further described below.
The second polymeric component may also be any other
polymeric component described elsewhere herein.
Metal
The metal or metal components used in the devices may be any
suitable metal which is biocompatible.
In a preferred embodiment the metal component is selected
from one or more of the metals titanium, gold, silver, chromium-cobaltum,
zirconia, cobalt-chromium- molobdenum alloy and/or a ceramic of one or more of
these metals and alloys and may be of any structure as described elsewhere
herein.
More preferred are titanium, gold, silver and/or
chromium-cobaltum,
The metal components of the device may be in the form of
fibre or filaments, although in this text "fibre" means both fibre
and filaments. The metal components may also be in the form of powder, which in
this text meand powder or pellets or chopped fibres.
The Cobalt-Chrome alloys may be with the base metals cobalt
and chrome mixed with smaller quantities of other metals.
The amount of cobalt in a cobalt-chrome alloy may be at
least 25%, such as at least 30%, such as at least 35%, such as at least 40%,
such as at least 45%, such as at least 50%, such as at least 55%, such as at
least 60%, such as at least 65%. The amount of chrome in a cobalt-chrome alloy
may be at least 10%, such as at least 15%, such as at least 20%, such as at
least 25%, such as at least 30%, such as at least 35%, such as at least 40%,
such as at least 45%, such as at least 50%, such as at least 55%, such as at
least 60%, such as at least 65%.
Any combination of the amounts mentioned above of cobalt and
chrome which in total do not exceed 100% may be used as a metal in a device
according to the present invention.
Preferred is an alloy with at least 34% cobalt, at least 29%
chrome mixed with smaller quantities of other metals. Optionally nickel may be
one of the other metals.
Preferred is the alloy named Vitallium e.g. in the
combination of 60% cobalt, 20% chromium, 5% molybdenum, and traces of other
substances.
Titanium alloys may be used as part of a device, in such
alloys the base metal is titanium.
Preferred is a titanium alloy with aluminium. The aluminium
amount may be 1-20%, e.g. 1-10 %, such as about 4%.
Stainless Steel alloys may be used as part of a device, in
such alloys the base metal is iron, mixed with larger quantities of chrome and
nickel and some other metals
Preferred is a stainless steel alloy with iron in an amount
of at least 40%, such as at least 50%, such as at least 60%, such as at least
70%.
Preferred is a stainless steel alloy with at least 58% iron,
mixed with larger quantities of chrome and nickel and some other metals.
The metal can be used in any form, like powder, granulate,
chopped fibres, long fibres, 2D structural components like plates, 3D
structural components like shaped plates or hemicircles with holes. Also a
combination of these forms may be used. In different areas of a device
different metals and/or different alloys may be used, e.g. in a layered
structure comprising three polymer layers each further comprising one or more
metal component, each polymer layer may comprise e.g. fibers etc. of different
or similar metal or alloys.
Fabric
From the longer polymer fibre comprising the first and/or
the third polymeric component as previously described a fabric may be
constructed. Optionally this fabric may also comprise metal fibre.
A fabric may constitute the upper and lower layers of a
medical device.
The second polymeric component, optionally comprising one or
more metal components, may also be in the form of a fabric. Preferred is a
fabric of UHMWPE fibre. The fabric corresponds to the first and/or third
polymeric component as described elsewhere herein.
Within the fabric, the first polymeric component and the
third polymeric component are preferably in the form of fibre. Methods of
construction of fibres are known to persons skilled in the art. The polymers
may be aligned and/or spun into fibre by gel spinning or filaments, which again
may be spun into strands. From the fibres and/or filaments and/or strands the
layers of polymeric materials may be manufactured.
Metal fibre may also be treated to produce filaments and/or
strands. The methods are known to persons skilled in the art.
The fabric may be produced into a suitable shape, the shape
is preferably constructed by weave, knit, crochet, stitch, plait, interlace,
intertwine, interlock, link or unite the fibre and/or filaments and/or strands
of polymeric and/or metal fibre in other ways such as non-woven techniques.
Preferable the fabric is woven or knitted.
In an embodiment the fabric can be woven using different
techniques, the techniques include but are not limited to cord woven, linen
woven, mat woven, Celtic woven and twill woven. Persons skilled in the art know
variations of these techniques, the variations is hereby incorporated.
According to an embodiment of the invention the polymer
and/or metal fibres are woven into a squared fabric comprising intercepts with
angles of 90 degree. The
dimension and weaving style of the fabric is optional, preferred is a binding
style of 3:1 (twill). The fabric can if the thickness allows it be
rolled into a roll, from which suitable pieces are detached before the
stratified polymer product is constructed.
Products which can be used comprises but is not limited to
fabric of Dyneema® from DSM, Spectra® from Allied Signal Inc. Preferably the
fabric is workable in the process of construction of the medical device as
described elsewhere herein.
In another preferred embodiment the fibres, filaments or
strands of the constitution described above are woven into the fabric in a
shape suitable for the shape of the polymeric product. The shape of the fabric
can be any possible shape including but not limited to round, oval, triangle,
quadrangle, square, rectangular, pentagon, hexagonal etc. and may be
symmetrical or asymmetrical in any direction. Preferred shapes of the fabric
are quadrangle and round.
In one embodiment the polymer and/or metal fibres in each
layer of the fabric are positioned over each other making a structure wherein
the angles of the intersect are of 1 to 179 degree, such as in angles of 40 to
150 degree, for example such as in angles of 60 to 130 degree, such as in
angles of 70 to 110 degree, for example such as in angles of 80 to 100 degree,
such as in angles of about 90 degree. Most preferred is intersects of fibre and
strands in angles of about 90 degree.
The thickness of the fabric is preferably determined by
thickness as well as the number of fibres and/or filaments and/or strands and
the distance between these fibres, filaments and strands in the fabric. The
overall thickness of the fabric is preferably between 0.001 mm and 3 mm,
preferred is between 0.01 mm and 2 mm, more preferred is between 0.02 mm and
1.5 mm, further preferred is between 0.03 mm and 1.0 mm, yet further preferred
is between 0.04 mm and 0.08 mm, most preferred is between 0.05 mm and 0.06 mm.
In an embodiment the area weight of the fabric is preferred between about 10
g/M2 and 500 g/M2 preferred is an area weight of between about 50 g/M2 and
300 g/M2, more preferred is an area weight of between about 75 g/M2 and
250 g/M2, further preferred is an area weight of between about 100 g/M2 and
200 g/M2, yet more preferred is an area weight of between about 125 g/M2 and
175 g/M2, even more preferred is an area weight of between about 140 g/M2 and
160 g/M2, most preferred is an area weight of about 150 g/M2.
In an embodiment the thickness and/or area weight of the
fabric varies across a single sheet of fabric. Hereby the area weight of the
fabric also varies in the device.
The area weight of a single fabric vary at least 5% across
the fabric sheet, such as
10%, such as 20%, such as 30%, such as 40%, such as 50%,
such as 60%, such as 70%, such as 80%, such as 90%, such as 100%, such as 120%,
such as 140%, such as 160%, such as 180%, such as 200%, such as 250%, such as
300%, such as 350%, such as 400%, such as 450%, such as 500% according to the
area weight of the thinnest 1 cm2 of the fabric.
In an embodiment the thickness of the fabric has a first
thickness in the middle area of the device and a second thickness in at least a
part of the outer area of the device. The first thickness of may be smaller
than the second thickness, and can vary as described above.
The fibres, filaments and strands from which the fabric is
produced according to the description herein, may have a fibre diameter
preferably between 100 and 650 dtex. The fibre diameter of the warpyarn is
preferably about 300-650 dtex, more preferably about 350-550 dtex, further
preferably about 400-500 dtex, most preferably about 430-460 dtex. The weftyarn
is preferably about 100-350 dtex, more preferably about 150-300 dtex, further
preferably about 175-250 dtex, most preferably about 210-230 dtex.
The fabric need not be constructed of fibre or filaments or
strands with equal thickness. A woven fabric where some of the strands have a
larger thickness than the rest may be used. In this way e.g. every second,
every third or more strands in between may have a larger thickness than the
rest of the strands of the fabric. The fabric described herein may also be
constructed by strands of different polymers and/or of different metals. The
different polymers and/or of different metals may be selected among the
polymers and metals listed herein above. Two or more polymers and/or metals may
be used in the construction of the fabric.
In an embodiment the thickness of the fabric may vary
according to different thickness of the polymer and/or metals strands as
described above or different polymers and/or metals may be utilised to
construct the fabric. Also different numbers of strands pr cm may be used.
In an embodiment the surface dimension of one or more inner
layers of fabric may be smaller than the total surface dimension of a medical
device. Smaller layers of fabric may enclose inlays.
In another preferred embodiment the fabric has a high
tensile strength and a high wear resistance. The degree of tensile strength is
determined by the polymer utilised to produce the fibre and the thickness of
the fibre. The tensile strength of the strand or fibre in a fabric is
preferably above 1.0 GPa, such as above 1.2 GPa, preferable above 1.4 GPa, more
preferable above 1.6 GPa, further preferable above 1.8 GPa, yet further
preferable above 1.9 GPa, most preferable above 2.0 GPa.
In another embodiment the tensile strength of the strand or
fibre in a fabric is preferably above 0.05 GPa, such as above 0.1 GPa,
preferable above 0.3 GPa, more preferable above 0.5 GPa, further preferable
above 0.7 GPa, yet further preferable above 0.8 GPa, most preferable above 0.9
GPa.
Although the term 'fibre' is used in the description of
fabric comprising the first and third polymeric components, filaments and/or
strands and/or other components comprising long chains of polymer units may be
used instead of fibres.
The fabric constitutes a reinforcement fabric or tissue of
the device.
Film, core and inlay.
If the
device of the present invention is constructed as a laminated device, the
middle layer of the polymeric product may comprise a second polymeric component
optionally further comprising one or more metal components. The
polymeric component optionally comprising one or more metal components may be
any short chain polymer material or low density polymer material as described
above. Also chopped strands of long chain polymer material such as fibre and/or
filaments and/or strands may be utilised as short chain polymer materials.
Preferred is when the chopped strands comprising short chain polymers are
moulded into a matrix with low density polymer material or a polymer comprising
the second polymeric component as described elsewhere herein.
By 'chopped strands' is meant shorter chains or strands cut
from fibres and/or filaments and/or strands.
In an embodiment the middle layer comprising polymer layer
comprises a film, a core or an inlay.
The polymer layers 'film', 'core' and 'inlay' may be
produced of similar or substantially similar or different polymers optionally
comprising metal components. Preferred are polymer layers of film, core and
inlay which are produced by similar polymers optionally comprising metal
components. Polymers suitable to be used are described above.
The differences of film, core and inlay may be the
dimensions of the polymer layers. The dimensions are determined according to
the function of the polymer layers. The film, core and inlay may differ in
thickness from each other, but may also have similar thickness, whereby film
and core sometimes can substitute each other in the composition of the medical
device.
The visual difference of film and core is preferably based
on the thickness, where the film in general is thinner than the core. The main
purpose of a film layer is to attach two layers of fabric to each other, and
simultaneously provide the device with characteristics such as capability of
absorbing shocks, impacts and pressure load. The core may also attach fabrics
to each other, and provide the same characteristics to the device as the film,
but the core may be utilised in devises subjected to higher degree of impacts
and pressure load than to devises comprising no core layer.
The difference of core and inlay may be based on the length
and width of the polymer layers optionally comprising metal components, the
inlay may be smaller than a core. The function of an inlay is to absorb shocks
and pressure in specific areas of a medical device. An inlay of one device may
be larger than a core of another device.
In an embodiment the middle layer comprises a film or core
or inlay. The film and core comprises the polymers described above optionally
comprising metal components, and may be constructed by melting the polymers
optionally comprising metal components. Mixtures of polymers may be used to
construct the film, core or inlay. The melted polymeric mass may be formed
according to any method possible, the methods are known to persons skilled in
the art. The methods comprises but are not limited to blow moulding, extruding,
foil moulding, injection moulding, compression moulding, preferred is blow
moulding. Preferred methods are moulding of the melted polymeric mass in small
or large open moulds/vats or injection moulding, the thickness of the material
is optional, but is chosen not to be changed followed solidification. Following
solidification the solidified polymeric matrix optionally comprising metal
components can be cut or punched or stamped out to a suitable dimension.
The suitable dimension of the film may be determined in
accordance to the scope of the application. The preferred application of the
film is as a thin polymer layer optionally comprising metal components between
two layers of fabric, in this situation the size comprising length and width of
the film is at least the length and width of the polymeric material used to
produce a medical device, hereby the film may be squared, circular or any other
dimension as any surplus of polymer material is removed following formation of
the medical device.
In an embodiment the device is constructed from layers of
fabric, film, core and/or inlay where the layers each has a dimension suitable
to construct the device without any process of removing surplus of polymer
layers. In this process the polymer layers of film, core and/or inlay may have
dimensions smaller than the outermost layer of fabric. To adjust the size of
the polymer layers to the form of the device to be produced, inner layers of
fabric may be smaller than the outermost layer of fabric. The outermost layer
of fabric which constitutes the inner side of a medical device may also be
smaller than the outermost layer of fabric which constitutes the outer side of
a medical device.
The suitable dimension of the core may also be determined in
accordance to the scope of the application. The preferred application of the
core is as a polymer - optionally comprising metal components - layer between
two layers of fabric, where the core fills in all the area comprising length
and width between the two layers of fabric, in this situation the size of the
core is at least the length and width of the polymeric material used to produce
a medical device, hereby the core may be squared, circular or any other
dimension as the surplus of polymer material is preferably removed following
formation of the medical device.
The suitable dimension of the inlay may also be determined
in accordance to the scope of the application. The preferred application of the
inlay is as a polymer layer optionally comprising metal components, which fills
in part of the area between two layers of fabric or film; hereby the inlay may
comprises any dimension appropriate for the purpose of the medical device. The
inlay is moulded into the appropriate dimension or it is cut into the appropriate
dimension.
The preferred thickness of the core and of the inlay is
chosen in accordance with a reduction of the thickness in the construction of
the device. During the pressing process the thickness of the core and the inlay
may be reduced by up to 50%, as the short chain polymers of the inlay and/or of
the core are pressed in between layers of fabric. By this pressing process the
other dimensions comprising length in two dimensions of the core and inlay may
increase as the thickness decreases.
Objects of non-polymeric material or objects of polymeric
material different to the material which the core or inlay is made of, may be
placed within core or inlay. The objects may be but is not limited to metal
globes or metal sheets. The objects may be incorporated in the inlay or core in
the moulding process or may be placed in holes made in the inlay or core in the
moulding process or made afterwards. An example of objects in an inlay is metal
globes in the inlay. These objects are different in composition and/or material
type than the metal components described above used in the production of the
core or inlay.
The difference of film and core has a fluid borderline,
whereby the utility of film and core may be interchangeable. Also the
difference of core and inlay has a fluid borderline, whereby the utility of
core and inlay may be interchangeable
In an embodiment the film is prepared as described
elsewhere, the film is preferably between 0.001 and 5 mm thick, such as between
0.01 and 5 mm, preferable between 0.1 and 4 mm, more preferable between 0.2 and
3 mm, further preferable between 0.3 and 2 mm, yet further preferable between
0.4 and 1.5 mm, most preferable between 0.5 and 1 mm.
The core or inlay which are also prepared as described
elsewhere, is preferably between 0.1 and 30 mm thick, such as between 0.2 and
25 mm, preferable between 0.3 and 21 mm, more preferable between 0.4 and 17 mm,
further preferable between 0.5 and 13 mm, yet further preferable between 0.6
and 10 mm, most preferable between 0.7 and 7 mm.
In an embodiment the surface dimension of one or more layers
of film may be smaller than the total surface dimension of a medical device.
Smaller layers of film may be used on one or more sides of smaller size fabric.
In a preferred embodiment the polymers as described above
are of medical grade.
The film, core and inlay may comprise short chain polymers.
Examples of characteristics, properties and additives of the short chain
polymers are shown in the following tables
Mechanical
properties measured on a moulded plaque.
More preferred examples of characteristics, properties and additives
of the short chain polymers are shown in the following tables
Mechanical
properties measured on a moulded plaque.
Processing may be performed at an advised temperature of 150C
to 180C.
Additives may be used in the short chain polymer material,
preferred is none slip agent and none anti-blocking additives.
An example of a short chain polymer material is Lacqtene® FE
8000 from Atofina.
The polymeric material of the film, core or inlay may include
polymeric and/or metal fibres with a uniform length or with varying length. The
fibres can be nano-fibres or chopped fibres. The material can also be a
composite material e.g. of the type as described elsewhere herein. Further the
material can be armoured polymers. In a nano-structured composite short and/or
long composite material can be utilised.
The film, core or inlay may be produced by moulding, such as
injection moulding, or injection extrusion. By controlled injection extrusion
it is possible to control the crystal number and placing.
Production of a layered polymeric product
In one embodiment the polymeric product optionally
comprising one or more metal component from which medical devices are
constructed comprises polymer layers in a sandwich or laminated format with at
least three polymer layers, where the polymer layers are fused together by a
heating process. Each of the polymer layers may optionally comprise one or more
metal component. The middle or at least one inner layer may differ from the two
or more outer layers, hereby the polymer layers may constitute a film, or a
core or an inlay with at least one layer of fabric on each side. The fabric
provides a high wear resistance and high tensile strength, while the inlay and
core and to some degree also the film absorbs shocks.
In the production of a medical device, the different polymer
layers as described above may be laminated in accordance to the required
characteristics of the medical device. In an embodiment the middle or at least
one inner layer of the polymeric product may constitute a core or a film, on
each side of the core or film a fabric is positioned. In a preferred embodiment
the polymeric product are composed of three layers where the fabrics at the
different sides of the core have equal constitutions. Each of the polymer
layers may optionally comprise one or more metal component.
The layers of fabrics within a device can be different
according to the polymers and/or the metals utilised to produce the fabrics or
the fibres, or strands within the fabrics may be different, or the fabrics are
produced in different ways, also the fabrics can have different thickness. In a
cup shaped device the outer part of the cup may comprise a thicker fabric than
the inner part of the cup, hereby increasing the wear resistance of the outer
part. In another embodiment the polymeric product are composed of more than
three layers, where, in between two fabrics a film or a core or an inlay are
positioned. The individual layers of fabric may be substantially identical,
identical or different in composition. Also the layers of film, core and inlay
may be substantially identical, identical or different in composition.
In an embodiment the number of the layers core, film, inlay
and fabrics differ across the polymeric product. The number of the layers can
also vary in different areas of the polymeric product. The outermost layer of
each side of the product must be a fabric, and two layers of fabrics have a
film or a core or an inlay in between. With varying number of layers across the
polymeric product, also the thickness of the product varies. Some areas may
contain an inlay other areas may be without the inlay.
In a further embodiment the outermost layer of the product
can be a film. The film is positioned next to a layer of fabric. Both sides of
a product may have film layers as the outermost layers or only one side is a
film layer. In case the medical device has more than two outer sides, one or
more sides may be a film layer.
The polymeric product optionally with one or more metal
components can also constitute two or more layers of fabrics on each side of a
core or an inlay, and the two or more layers of fabrics may have a film of a
polymer layer optionally with metal in between each fabric. Following heating
of the layered polymeric product the film and/or core and/or inlay mechanically
connect or bond together two layers of fabric.
Layers of film and/or core and/or inlay in a device may be
different according to the polymers utilised to produce the layers. The
polymers may be of different types, preferred polymers are mentioned herein
above. Also the polymers may be mixtures of different types of polymers or
mixtures of polymer chains of different length or both. Each of the polymer
layers may optionally comprise one or more metal component.
Film may have a higher adhesiveness than core and inlay. The
number of layers of fabric in a medical device is optional, as well as the
number of layers of film and fabric and inlay can differ on each side of a core
or of an inlay. The number of layers of fabric in a medical device is
preferably between 1 and 100, such as between 2 and 50, for example between 2
and 40, preferable between 2 and 35, more preferable between 2 and 30, further
preferable between 2 and 25, yet further preferable between 2 and 20, most
preferable between 2 and 10.
Also the number of layers of film in a medical device is
optional, the number of layers of film is preferably between 0 and 100, such as
between 1 and 50, for example between 1 and 40, preferable between 1 and 35,
more preferable between 1 and 30, further preferable between 1 and 25, yet
further preferable between 1 and 20, most preferable between 1 and 10.
In addition the number of layers of core in a medical device
is optional, the number of layers of core is preferably between 0 and 100, such
as between 1 and 50, for example between 1 and 40, preferable between 1 and 35,
more preferable between 1 and 30, further preferable between 1 and 25, yet
further preferable between 1 and 20, most preferable between 1 and 10. The
number of layers of film, inlay and fabric can be different at each side of a
core.
The number of layers of inlays in a medical device is
optional, the number of layers of inlay is preferably between 0 and 100, such
as between 1 and 50, for example between 1 and 40, preferable between 1 and 35,
more preferable between 1 and 30, further preferable between 1 and 25, yet
further preferable between 1 and 20, most preferable between 1 and 10. The
inlay can be positioned anywhere within the stratified polymer product between
two layers of film or fabric. The inlay may be smaller than the entire area of
the medical device, and the inlay may be located at any position within the
medical device. Also the number of layers of film, core and fabric can be
different at each side of an inlay.
Preferred layered compositions of the polymeric product of
medical devices comprises but are not limited to the constitutions:
• fabric - film - fabric.
• fabric - core - fabric.
• fabric -
film - core - film - fabric • fabric - film - fabric - core - fabric - film -
fabric.
• fabric - film - fabric - film - fabric - core - fabric -
film - fabric - film - fabric.
• fabric - film - fabric - film - fabric - film - fabric -
core - fabric - film - fabric - film - fabric - film - fabric.
• fabric -
film - fabric - film - fabric - film - fabric - film - fabric - core - fabric -
film - fabric - film - fabric - film - fabric - film - fabric.
• fabric - core - fabric - film - fabric - film - fabric.
• fabric - film - fabric - core - fabric - film - fabric -
core - fabric - film - fabric.
• fabric - film - fabric - film - fabric - film - fabric -
core - fabric - film - fabric.
• fabric -
film - inlay - film - fabric.
• fabric - film - fabric - film - inlay - film - fabric -
film - fabric.
• fabric - film - inlay- film - fabric - film - fabric -
film - fabric.
• fabric - film - inlay- film - fabric - film - inlay - film
- fabric.
• fabric - film - inlay - film - fabric - film - fabric -
film - inlay - film - fabric.
• fabric -
film - inlay - film - fabric - film - inlay - film - fabric - film - fabric.
• fabric -
film - inlay - film - fabric - film - inlay - film - fabric - film - inlay –
film - fabric.
• fabric -
film - inlay - film - fabric - film - fabric - film - inlay - film - fabric –
film - inlay - film - fabric.
• fabric -
film - inlay - film - fabric - film - fabric - film - fabric - film - inlay –
film - fabric - film - inlay - film - fabric.
• fabric - film - inlay - film - fabric - film - inlay -
film -fabric - film - inlay - film - fabric - film - fabric.
• fabric - film - fabric - inlay - fabric - film - fabric.
• fabric -
film - fabric - film - fabric - inlay - fabric - film - fabric - film - fabric.
• fabric - film - fabric - inlay - fabric - film - fabric -
film - fabric - film - fabric.
• fabric - film - fabric - inlay - fabric - film - fabric -
film - fabric - inlay - fabric - film - fabric.
• fabric - film - fabric - inlay - fabric - film - fabric -
film - fabric - film - fabric - inlay - fabric - film - fabric.
• fabric - film - fabric - inlay - fabric - film - fabric -
film - fabric - inlay - fabric - film - fabric - film - fabric.
• fabric - film - fabric - inlay - fabric - film - fabric -
film - fabric - inlay - fabric - film -fabric - film - fabric - inlay - fabric
- film - fabric.
• fabric -
film - fabric - inlay - fabric - film - fabric - film - fabric - film - fabric
- inlay - fabric - film - fabric - film - fabric - inlay - fabric - film -
fabric.
• fabric - film - fabric - inlay - fabric - film - fabric -
film - fabric - film - fabric - film - fabric - inlay - fabric - film - fabric
- film - fabric - inlay - fabric - film - fabric.
• fabric - film - fabric - inlay - fabric - film - fabric -
film - fabric - inlay - fabric - film - fabric - film - fabric - inlay - fabric
- film - fabric - film - fabric.
• film - fabric - film - fabric - film.
• film - fabric - core - fabric - film.
• film -
fabric - film - core - film - fabric - film.
• film - fabric - film - inlay - film - fabric - film.
• fabric - film - fabric - film.
• fabric - core - fabric - film.
• fabric - film - core - film - fabric - film.
• fabric -
film - inlay - film - fabric - film.
• fabric - film - fabric - inlay - fabric - film - fabric -
film.
In this list the layers may be made of a polymer optionally
further comprising one or more metal components.
The design
of the device as listed above may be combined with one or more other features
as described herein, e.g. in the design one or more of the mentioned fabric,
film, core and/or inlay may comprises metal in any of the compositions as
mentioned elsewhere herein In case two similar layers are used in the device
e.g. two layers of fabric, these two layers need not be of similar materials or
have similar properties.
The mentioned layers need not be visible in the product
ready to use. The layers may also be relevant only in respect of the polymeric
components used, and one or more different metals may be distributed throughout
or substantially throughout the device. Preferred is when the at least one of
the outermost layers comprises less than 50% metal at the surface, such as less
than 40%, e.g. less than 30%, such as less than 20%, such as less than 10%.
In the preferred embodiments described in the list above
fabric comprises the first and/or third polymeric component as described
elsewhere herein, and film, inlay and core comprise the second polymeric
component as described elsewhere herein. The polymeric layers may each further
comprise one or more metal components.
The layers of fabrics within a device may be similar or may
be different in the construction. Also one or more layers of fabrics within a
device may differ from the other layers of fabrics. Similar situations can be
obtained regarding the film, inlay and core. Film, inlay and/or core of a
single device may be different in construction.
By 'different in construction' is meant that the layers of
interest can be produced by different materials or partly by different
materials or the process of manufacture is different thus giving the layers
different properties.
The constitutions of a product mentioned above may be
surface coated by plasma polymerisation.
The polymeric material as described herein optionally
further comprising one or more metal components can also be used to cover
prostheses of other materials, such as standard prostheses.
In another aspect of the invention the medical device
comprises one or more layers of fabrics which may be surface coated by plasma
polymerisation.
Features of the product
The thickness of the polymeric product is determined by the
number of polymer layers optionally comprising one or more metal components and
the dimension of these layers in accordance to the requirements of the medical
device. The total thickness of the polymeric product is preferably between
0.001 and 40 cm thick, such as between 0.005 and 30 cm, preferable between 0.01
and 20 cm, more preferable between 0.02 and 10 cm, further preferable between
0.03 and 8 cm, yet further preferable between 0.04 and 5 cm, most preferable
between 0.05 and 2 cm. In another embodiment the preferred thickness of a
device is about 3 mm.
The surface area of a medical device may be between 1 cm2 and
200 cm2. The surface dimension of a medical device comprising the polymeric
layered structure as described herein may be between 0.01 to 40 cm according to
length and width, such as between 0.05 to 35 cm, for example between 0.09 to 30
cm, preferable between 0.1 to 25 cm, more preferable between 0.2 to 23 cm,
further preferable between 0.3 to 19 cm, yet further preferable between 0.4 to
17 cm, most preferable between 0.5 to 15 cm. Other preferred sizes of the
surface dimension of a medical device may be between 0.5 to 8 cm according to
length and width, such as between 0.5 to 7 cm, for example between 0.5 to 6 cm,
preferable between 0.5 to 5 cm, more preferable between 0.5 to 4 cm, further
preferable between 0.5 to 3 cm, yet further preferable between 0.5 to 2 cm,
most preferable between 0.5 to 1 cm.
The surface dimension according to length and width of
layers of fabric in a medical device as described herein may be substantially
equal, equal or different from the surface dimension of the medical device. Preferred
is a size where any surplus of fabric is removed following manufacture of the
medical device.
To enclose one or more inlays with fabrics, the size of
fabric according to the surface dimensions length and width may be the same as
for the inlay, substantially the same as for the inlay or somewhat larger than
the inlay. One or more inlays may be enclosed by two or more layers of fabric.
The layers of fabric may have surface dimensions adjusted to cover all the
inlays, although the inlays may have distance between each other. Two or more
inlays of a device may or may not be positioned in between the same two layers
of fabric.
The surface dimension according to length and width of
layers of film in a medical device as described herein may be substantially
equal, equal or different from the surface dimension of the medical device.
Preferred is a size where any surplus of fabric is removed followed manufacture
of the medical device.
The surface dimension according to length and width of
layers of core in a medical device as described herein may be substantially
equal, equal or different from the surface dimension of the medical device.
Preferred is a size substantially equal to the surface dimension of the medical
device. The surface dimension according to length and width of layers of inlay
in a medical device as described herein may be substantially equal, equal or
different from the surface dimension of the medical device. Preferred is a size
where the inlay is smaller than the surface dimension of the manufactured
medical device.
To increase the strength of the polymeric product, the
layers of fabric may be turned according to each other, hereby the fibres of
the different layers of fabric is positioned into different directions. The
fabric may be turned between about 0 to about 90 degree, such as between 10 and
80 degree, preferred is between 20 and 70 degree, more preferred is between 30
and 60 degree, further preferred is between 38 and 52 degree, yet further
preferred is between 42 and 48 degree, most preferred is about 45 degree in
relation to the former and/or next layer of fabric.
In an embodiment at least part of the polymeric component or
polymeric compo- nents optionally comprising one or more metal components , of
the device, is suitable for cells to grow into it. Preferred is when the
surface of the device is in a condition suitable for cells to enter and grow
into this surface. This in-growth of cells secure the device within the joint
of the mammal.
The medical device may be secured with a collar and can be
of any design and construction as mentioned elsewhere herein.
Method for preparation
Polymers may be prepared by methods known to the person
skilled in the art.
Chemical catalysis, thermal induction or photo induction are
anecdotal non-limiting examples of methods of preparing the polymers.
The polymeric product optionally further comprising one or
more metal components may be prepared according to the descriptions above by
connecting stacked polymer layers by heating. The heating temperature is
selected below the melting temperature of the fibre crystallites in order not
to loose the crystallinity of the polyethylene fibres and to a level where the
fibres of the fabrics are not melted, destroyed or damaged, ie. below 250
degree Celsius, and above the melting temperature of the polyethylene
plastomer, ie. in the range between 80 and 250 degree Celsius, such as between
90 and 240 degree Celsius, preferable between 100 and 230 degree Celsius, more
preferable between 110 and 220 degree Celsius, further preferable between 120
and 210 degree Celsius, yet further preferable between 130 and 200 degree
Celsius, most preferable between 140 and 190 degree Celsius.
In an embodiment the heating temperature is preferably
between 90 and 200 degree Celsius, such as between 95 and 195 degree Celsius,
preferable between 100 and 190 degree Celsius, more preferable between 105 and
185 degree Celsius, further preferable between 110 and 180 degree Celsius, yet
further preferable between 115 and 175 degree Celsius, most preferable between
120 and 170 degree Celsius.
In a further embodiment the heating temperature is
preferably between 90 and 180 degree Celsius, such as between 95 and 170 degree
Celsius, preferable between 100 and 160 degree Celsius, more preferable between
105 and 155 degree Celsius, further preferable between 110 and 150 degree
Celsius, yet further preferable between 115 and 145 degree Celsius, most
preferable between 120 and 140 degree Celsius
When heated the short chain polymers of the core or the film
or the inlay penetrate into the fibres or filaments or strands of the fabrics,
and hereby mechanically connect or bond the polymer layers to each other. In a
preferred embodiment the temperature is selected to a level where the main part
of the fabrics is not melted, but a thin layer constituting a low number of
polymer chains or fibres of the outer part of the outermost fabrics of the
polymeric product is melted. The heating process may be provided in vacuum and
under pressure.
In a further preferred embodiment the temperature is
selected to a level where the performances of the reinforcement fibres are not
damaged.
The polymer product which may comprise stratified polymer
layers as described above, and which has been subjected to the heating process,
may be stored at room temperature until use. The polymeric product optionally
comprising one or more metal components is preferable capable of being stored
for long periods of time, such as several years. Storing is performed in dry
conditions at room temperature and in darkness or at least without direct sunshine
to the product. Dry conditions may be humidity of about 10-90 %.
The medical device prepared by the described method can be
of any design and construction as mentioned elsewhere herein.
Shaping the medical device
In the heating process described previously or following a
storage period of the fused polymer layers, the polymeric product comprising
the polymer layers may be subjected to vacuum and may simultaneously be pushed
or pressed into a mould to form the polymeric product. The vacuum process prevents
the formation of bubbles and protects the polymer from oxidative degradation.
Shaping can also be performed without vacuum but due to pressure optionally
combined with heating the polymeric product.
The vacuum of the method described above may be below 500
mbar, preferable below 300 mbar, more preferable below 100 mbar, further
preferable below 50 mbar, yet further preferable below 10 mbar, most preferable
below 1 mbar.
To secure the desired shape of a device, the pressure of the
device in the shaping process may be maintained until the polymer product is
cooled preferable to room temperature. This cooling under pressure secures
consolidation of the product.
The pressure in the process described above is a pressure
high enough to press the product into a mould, the pressure may be a low
pressure performed for a long period or a high pressure performed for a short
period, or a pressure in between. Low pressure in this context is the pressure
just enough to press the product into a mould.
The polymer product optionally comprising one or more metal
components pressed into a shape as described above, may be stored at room
temperature for long periods of time, such as several years. In an embodiment
the polymeric product optionally comprising one or more metal components may be
produced with one or more apertures, holes, gaps, perforations or hollows. The
apertures etc. may constitute an improved attachment and/or optimise the
function of the device. The improved attachment may be obtained without further
processing as the apertures may constitute a shape of the device in a way that
the device better fits into the location of the body. The apertures can also be
utilised to fasten the device within the body.
Fastening methods are known to persons skilled in the art,
and are hereby incorporated.
The apertures etc. may be created simultaneously with the
shaping of the polymeric product, hereby the mould has points, tips or peaks,
which create the apertures in the polymeric product. Another method of
producing apertures etc. is to make a hole by a drill or another boring,
cutting or pressing apparatus. Followed the formation of apertures etc. by
drilling, cutting or pressing holes, the edge of the apertures on the polymeric
device may be closed.
To the polymeric material optionally comprising one or more
metal components may be attached a component, the component being polymeric or
non-polymeric. The attachment may constitute part of a prosthesis or provide an
anchorage point.
The medical device of the shape described can have of any
design and construction and be of any material as mentioned elsewhere herein.
Composition of devices
The device may be layered only with polymer layers
optionally comprising one or more metal components, or a combination of metal
layers together with polymer layers optionally comprising one or more metal
components.
In an embodiment the number of layers of polymer optionally
comprising one or more metal components may be similar to or lower than the
number of layers of metal. The composition may be according to the following
list, where polymer means a polymer layer optionally comprising one or more
metal components, The list is not exhaustive:
• metal - polymer
• metal - polymer - metal • metal - polymer - polymer -
metal
• metal - polymer - metal - polymer - metal
• metal - polymer - metal - polymer - polymer - metal
• metal - metal
• metal - metal - polymer • metal - metal - polymer -
polymer
• metal - metal - polymer - metal
• metal - metal - polymer - polymer - metal
• metal - metal - polymer - metal - metal
• metal - metal - metal
The list
does not indicate the orientation in respect of outer and inner or upper and
lower layer of the device. In a device with two or more polymer layers,
these layers can be of a similar composition or be different in composition. In
a device with two or more metal layers, these layers can be of a similar
composition or be different in composition.
In a device which comprises one or more layers of metal
located next to each other the metal layers may be only soft metals, only hard
metals or a combination of soft and hard metals.
In an embodiment the number of layers of polymer optionally
comprising one or more metal components may be similar to or higher than the
number of layers of metal. The composition may be according to the following
list, where polymer means a polymer layer optionally comprising one or more
metal components, The list is not exhaustive:
• polymer - polymer
• polymer - metal
• polymer - metal - polymer
• polymer - metal - metal - polymer
• polymer - polymer - metal - polymer
• polymer -
polymer - metal - polymer - polymer
• polymer -
metal - polymer - metal - polymer
• polymer -
polymer – polymer
Devices
produced from layered structures may be any devices as mentioned else- where
herein.
In an aspect of the invention the prosthetic device is not a
layered product, or not produced from layers of polymer and/or metal. The
device may be produced from on single polymer product optionally further
comprising one or more metal components or may be produced entirely or
substantially entirely from one type of metal or metal alloy.
In a device with a non-layered structure different areas of
the device may be made from two or more polymer.
In an embodiment the device is made of a polymer which is
suitable of injection moulding, to this polymer is added one or more types of
metal fibre and/or metal powder. Optionally also polymer fibre can be added.
Preferably the polymer which is suitable of injection moulding is one or more
polyolefin. More preferred the polymer is polyethylene and/or polypropylene.
Preferably the metal is selected from titanium, gold, silver and/or
chromium/cobaltum.
In an embodiment the device is produced by shaping a
polymeric material comprising fibre and/or powder of metal, optionally polymer
fibre may also be added to the polymer. The shaping may be performed by
injection moulding.
In an embodiment a device, e.g. an acetabular cup or an
interpositional arthroplasty can be made of a polymer which is suitable of
injection moulding, to this polymer is added one or more types of metal fibre
and/or metal powder. Optionally also poly- mer fibre can be added. Preferably
the polymer which is suitable of injection moulding is one or more polyolefin.
More preferred the polymer is polyethylene and/or polypropylene. Preferably the
metal is selected from titanium, gold, silver and/or chromium/cobaltum. In
another embodiment a device, e.g. an acetabular cup or an interpositional
arthroplasty can be made of a 3D network or a mat of metal fibre and/or polymer
fibre. The mat is filled with a polymer with a lower melting point than the
polymer fibre and the device is subjected to heat and pressure to produce a
device comprising fibre of metal and/or polymer fibre in a matrix of polymer.
The 3D-network may have a knitted, crochet and/or weaved
structure, or a structure described elsewhere herein.
The metal to be used in the 3D-network may be any suitable
metal. Preferred is a metal selected from titanium, gold, silver and/or
chromium/cobaltum.
The polymer fibre to be used in the 3D-network may be fibre
of any polymer described elsewhere herein. Preferred is a polymer fibre of
polyethylene or polypropyl- ene. More preferred is UHMWPE.
Devices produced without a layered internal structure or
without laying layers towards each other in the production process may be any
devices as mentioned elsewhere herein.
Collar
Following the shaping of the polymeric product optionally
comprising one or more metal components as described elsewhere herein, any
surplus of polymeric material can be removed e.g. by cutting off. Cutting off
the surplus of polymeric material leaves a polymeric product with right angle
edges. These edges have to be rounded to secure no damage of the product is
performed within the animal or human body when function as a medical device
within the body.
In an embodiment the rim of the device may be treated to fix
loose ends of fibre or strands. The rim may be closed by sewing or by fastening
a polymer ring or a metal ring. When using a ring to close the rim of the
device, the ring may be 0.5-5 mm thick, preferred is 3 mm. In another
embodiment a collar is placed on the medical device when surplus of material is
removed. The collar can be moulded directly on the device e.g. by injection
moulding.
In another embodiment a collar is moulded before positioned
onto the edge or edges of the device. Preferred is when the collar is injection
moulded. The collar can be secured to the device by ultrasound welding, gluing,
sewing and/or laser welding.
The collar as described above can be of any material
mentioned in the description of the first, second or third polymeric component
optionally comprising one or more metal components. The collar material of the
medical device can be the same material or a different material as actually
used for the first, second or third polymeric component optionally comprising
one or more metal components. Preferred is when the collar includes fibre e.g.
of UHWMPE or LDPE. More preferred is collar of LDPE. Preferred is also when the
collar includes nano- structured composite of polymer and/or metal. Further
preferred is when the collar includes short and long composite of polymer
and/or metal. Most preferred is when the collar is produced of the same
polymeric component optionally comprising one or more metal components as
actually used for the core or film, due to compatibility between the materials.
Within the process the polymeric component of the collar may melt together with
the polymeric component of the film and/or core. Also preferred is when the
collar material includes nano-fibre or short fibre of polymers and/or metals.
In an
embodiment a cup shaped medical device where the hat brim of surplus of
material is removed a pre-moulded collar of LDPE optionally comprising one or
more metal components is attached on the cut edges.
The collar closes the edge, gives the cup strength, and
includes or support the marker e.g. in the form of a gold thread. Furthermore
the collar modify the friction.
Instead of mounting a collar on the edge of the device, it
is possible to bead or flange the edge or edges. This treatment can be
performed under heat. A collar that is pre-moulded before mounted to the edges
of a medical device is easier to handle, and has economical benefits compared
to injection moulding a collar onto the edges.
It can be difficult to mould a collar directly on the
medical device, as the device may become soft at the temperature and pressure
at which the collar is injection moulded onto the edge of the device. Hereby
there is a risk of deformation of the outer area of the device. With a
pre-moulded collar that is secured to the edge by ultrasound welding, gluing,
sewing and/or laser welding the edge and outer area of the device is not at
risk of the mentioned deformation.
The methods of fastening the pre-moulded collar to the
medical device secure a safer attachment between the collar and the edge and
the outermost part of the outer area of the device. The material of the collar
may adhere to both the first, second and/or third polymeric material of the
device, these polymeric materials optionally further comprise one or more metal
components. Preferred is ultrasound welding to attaching the pre-moulded collar
to the medical device.
The pre-moulded collar has a dimension that secure that the
collar when attached to the medical device covers at least 0.5 mm of the outer
area of the upper surface when measured from the edge. A similar dimension may
be covered at the lower surface of the device. The collar need not cover equal
dimensions of the upper and lower surface.
In an embodiment the pre-moulded collar has a groove wherein
the marker can be placed. Hereby the marker may be located at the edge of the
device and the collar encloses or support the marker Preferred is a thread or
wire of metal.
The marker can be in the form of one single unit or in the
form of at least two units, and where the at least two units are placed
non-homogeneous within the device.
Hereby the rotation of the device within the joint can be
detected as described elsewhere herein.
In an embodiment the collar includes and/or supports at
least one marker. These markers are described herein below. By including these
markers in the collar there is no need of placing marking particles in holes of
the device. Thus the incorporating of a marker in or supported by the collar
eliminates the step of drilling in the device.
In a preferred embodiment a cup-shaped or approximately
cup-shaped medical device has a top and a skirt with a thickness larger than
the thickness of the top, and where the edge of the skirt is enclosed by a
pre-moulded collar. The collar can include a marker as described elsewhere
herein.
Collars as described herein may be use for a medical device
of any design and con- struction as mentioned elsewhere herein.
Markers
In an embodiment markers are placed within the medical
device. The markers can be used to visualise, trace or in other ways show the
position of the medical device when inserted into a body. Visualisation can be
performed by methods known to a person skilled in the art, which is hereby
incorporated. One method is X-ray identification. The material of the markers
can be any material, which can be placed within the polymeric material
optionally comprising one or more metal components and can be detected from
outside of the body.
In one embodiment the markers are contrast balls. Before
closing the rim with a method described elsewhere, contrast balls are placed
within the device. The contrast balls which can be any suitable colour such as
but not limited to blue, red or green, can be placed in small holes drilled in
the device. The drilled holes can be made at a right angle to the surface
established when surplus of material is removed. Fastening a ring to the device
or moulding a collar as mentioned above closes the holes. The number of contrast balls are optional, in a
cup device 3-10 contrast balls may be utilised, 7 contrast balls is preferred.
In another
embodiment the markers are made of metal. The shape of the markers is
optional. Preferred are balls of metal. More preferred are markers of stainless
steel or tantalum. Most preferred are balls of stainless steel or tantalum. The
markers are placed within holes of the medical device drilled from the cut edge
appearing when surplus of material is removed. The number of markers is optional.
The number of markers is preferably 1-10, such as 2, e.g. 3, such as 4, e.g. 5,
such as 6, e.g. 7, such as 8, e.g. 9, such as 10. The placing of the markers
may be optional. Preferably the markers are placed asymmetrically around the
cut edge of the medical device. This asymmetric placement ensures the
possibility to measure if the medical device changes position when implanted in
the body. In a cup-shaped medical device the asymmetric placement of the
markers is preferable an asymmetric placement according to the circle
comprising the cut edge of the medical device, hereby it can be visualised
within the body whether the cup rotates. The cut edge is closed with a method
described elsewhere herein.
In a further embodiment the markers are small pieces of the
marking material. The small pieces are placed within the core or inlay when
these are moulded.
In another embodiment the markers are formed as threads or
wires, and are placed within the core, inlay, film or fabric when these are
produced. Threads of markers can also be placed between the fabric and any of
the core, inlay or film when the medical device is constructed from the
components.
The threads or wires may be of any material that is possible
to detect from the out- side of the body of a mammal. The material may be
metal, barium sulphate, Teflon, master batch, dye, contrast medium, or filler
in the collar for detecting the implant and the placement of the implant.
Examples of metal to be used as marker may be gold tantalum and titanium, which
can be used individually or jointly.
Preferred is when the marker is a metal thread or wire, and
the marker is non- homogenous placed in the cup, hereby it is possible to
detect rotation of the implant within the joint of the mammal.
Markers as described herein may be use for a medical device
of any design and construction as mentioned elsewhere herein.
The medical device with the marker may be detected by any
suitable detection method, such as X-ray, magnetic resonance scanning, CT
scanning, MRI scanning, or PET. Preferred is when the first, second or third
polymeric component optionally comprising one or more metal components or
another component of the collar is a X-ray sensitive component.
The marker is preferable not a magnetic marker when MRI
scanning, although a little magnetic character of the marker and/or device is
acceptable.
The marker can be a magnetic marker when X-ray detecting is
to be used. For X-ray detection the marker may include heavy atoms, and/or gold
octant.
In PET detection, electronic charged positrons are detected,
this result in inverted contrast when liquid is injected into the joint.
Fluorine NMR can be used to detect the device with Teflon
threads or Teflon wires, where the frequency of fluorine is utilised. In
Fluorine NMR it is only possible to observe the thread with fluorine. The
thread may include e.g. cilice, carbon, selenium.
Smoothness of the surface
The smoothness of the surface of the device is important as
this has a connection with the level of pain as well as a connection with pain
relief of the mammal in whom a medical device is located within a joint.
In an aspect of the invention the medical device comprising
at least a first and a second side, wherein the at least first side is made of
a first polymeric component, and wherein the first and/or second side has a
frictional resistance of less than 1 Newton. Preferred is less than 0.5 Newton.
More preferred is less than 0.2 Newton.
The device becomes more smooth when located in a joint and
this joint is in func- tion. The surface of the device will be exposed to some
wear, hereby the surface becomes more smooth. By producing a device which when
implanted is more smooth than normal, pain relief of the individual wherein the
device is implanted is obtained faster. In an embodiment the first polymeric
component has a frictional resistance of less than 0.5 Newton and the first
polymeric component is located in at least a first area where the device is
subjected to wear when the device has been implanted into an individual.
In an embodiment the wear in the at least first area is due
to friction made by a bone and/or a medical device.
In a joint in movement, the areas of the device which is
always subjected to contact with a bone or another part of an implant is
subjected to most wear.
In an embodiment the second side is made of a first and/or a
second polymeric component. The polymeric component of this second side may
have a similar or another smoothness as the component of the first side.
The surface
of the device can be made more smooth by moulding pressing the device an extra
time in succession to the preparation of the device. Another method to
improve the smoothness is to rub the device for a duration which is suitable to
obtain a desired smoothness.
In an embodiment the first and/or second side further
includes at least one cavity. The at least one cavity may have a diameter of at
least 0.05 mm when measured at the outside of the first side.
The cavities of the device provide more space for liquids such
as synovial fluid, physiological salt solution or another biocompatible
liquid.. Hereby is the friction coefficient lowered, the wear is lowered, a
lesser degree of pain is obtained by the mammal wearing the device and the
durability of the device is increased.
In an embodiment the at least one cavity has a depth of at
least 0.01 mm.
The cavities may be in the form of grooves. These grooves
may be in straight lines or in patterns e.g. waving lines or zigzag lines. In
one embodiment a device has a smooth surface together with cavities. Especially
the surface is smooth between the cavities in the areas which is subjected to
wear in the joint. Preferred is a cup-shaped device with a smooth surface and
cavities.
The medical device with the smoothness and cavities as
described can have of any design and construction and be of any material as
mentioned elsewhere herein.
Perforations
Medical
devices with perforations or apertures provokes an increased flow of liquids
such as synovial fluid within the joint.
In an aspect of the invention a medical device comprising at
least a first polymeric component, wherein the device has at least one
through-going perforation which is not for ligaments.
In an embodiment the at least one perforation has a diameter
of at least 0.01 mm.
In an embodiment the at least one perforation is located in
at least one area where the device is subjected to wear.
In an embodiment the perforations are located homogeneous
over substantially the entire device.
In an embodiment the perforations can conduct liquid from a
first side of the device to a second side of the device and/or from the second
side to the first side. Hereby the liquid can be displaced within the joint in
respect of the movement of the joint.
The liquid may be synovial fluid or physiological salt
solution or another biocompati- ble liquid.
In an embodiment the device further comprises an inner
volume between the first and second side and inside this inner volume the
liquid can be within. This volume may be an empty volume when the device is
inserted into the joint. Liquid within the joint can enter this volume. The
volume can also include liquid when inserted into the joint or be filled after
it is inserted. Hereby the inner volume comprises a storage of liquid.
The inner volume can comprise a network of at least one
polymeric component optionally comprising one or more metal components. This
polymeric component can be polymeric fibres and if present the metal components
can be metal fibre, where the empty space constitute space for liquid as
described above.
The medical device with the perforation and/or inner volume
as described can have any design and construction and be of any material as
mentioned elsewhere herein.
Self-healing characteristics
In an aspect of the invention a medical device comprises at
least a first polymeric component optionally comprising one or more metal
components, wherein the polymeric component is self-healing when subjected to
injury before implantation, during implantation and/or after implantation.
In an embodiment the polymeric component is a composite
polymeric component optionally comprising one or more metal components.
The device includes a self-healing polymer systems to
auto-repair cracks formed in plastics or composites materials. Microencapsulated
monomers (healing agent) and initiators are incorporated into a polymer matrix
to produce a polymer composite capable of self-healing. Cracks formed in the
material ruptures embedded microcapsules releasing healing agent into the
cracks. Polymerisation of the healing agent is triggered by contact with an
embedded catalyst, repairing the cracks. Alternatively the polymers comprising
the device can be selected from chemical structure bearing self healing
properties by being able to revert the chemical changes occurring upon rupture
In an embodiment the healing agent is monomers of the first
polymeric component and/or of a second polymeric component. The medical device with the self-healing
characteristics as described can have any design and construction and be of any
material as mentioned elsewhere herein.
Two congruent cups
In another aspect of the invention a medical device
comprises at least a first unit with at least a convex surface and a second
unit with at least a concave surface, where the convex and concave surface is
congruent with each other and the first unit fit partially or entirely into the
second unit, and wherein the first and/or second unit comprises at least one
polymeric component optionally comprising one or more metal components.
In an embodiment first and second unit have substantially
similar size.
In an embodiment the first and second unit have different
sizes.
In an embodiment the first and second units are connected or
substantially connected by the convex and concave surface. Hereby the one unit
fits into the other unit. The concave surface can be at least 0.01 mm smaller
than the convex surface.
In an embodiment the units are movable compared to each
other, and the units continue to be in contact during a movement and/or return
to be in contact when a movement is finished. The first unit and/or the second
unit may be attached to an implant and/or a bone.
In an embodiment the first unit and/or the second unit can
function without being attached to an implant and/or a bone.
In one embodiment the two units are two cup-shaped units,
which are secured or attached to a bone or implant or located at a bone or
implant or within a cavity. Between these cups may be located gas in the form
of air, liquid such as synovial fluid or a physiological salt solution or
another biocompatible liquid. The medical device with the two units, one with a
convex surface and the other with a concave surface as described can have any
design and construction and be of any material as mentioned elsewhere herein.
A fabric to be folded to constitute two polymeric layers
In an aspect o the invention a medical device comprises at
least an upper layer, a first middle layer and a lower layer, wherein the upper
layer and the lower layer is made from one uninterrupted piece of at least a
first polymeric component optionally comprising one or more metal components.
In an embodiment the piece of at least a first polymeric
component optionally comprising one or more metal components is in the form of
a fabric. The piece of at least a first polymeric component optionally
comprising one or more metal components is constructed in one piece, which is
folded around an axis and hereby forming the upper and lower layer.
In an embodiment the upper and lower layer are similar or
substantially similar in size. The upper and lower layer can also be of
different sizes.
In an embodiment the at least first polymeric component is
in the form of fibres.
In an embodiment the piece of at least a first polymeric
component is in the form of a tube or pipe before folded around the axis. The
tube may have a uniform diameter along the tube. Furthermore the tube may
contract in a first and/or a second end of the tube. The tube may also contract
in the axis.
In an
embodiment the piece of a first polymeric material optionally comprising one or
more metal components further comprises at least one aperture. This at
least one aperture may be located at the axis or in an area that is subjected
to a restricted amount of wear. The number of apertures is preferably between 1
and 20.
When the piece of a first polymeric material optionally
comprising one or more metal components is folded around the axis, the first
middle layer comprises empty space. This space may be filled with a second
polymeric component optionally comprising one or more metal components. One or
more of the apertures can be utilised to fill the device with a polymeric
component optionally comprising one or more metal components.
To the device a collar as described elsewhere herein may be
attached. The collar may include or support a marker as described elsewhere
herein. The marker can be a gold thread.
In an embodiment the fabric and hereby the tube is made by
weaving, knitting or crocheting.
In an embodiment the axis is located substantially at the
middle of the tube and the axis is parallel or substantial parallel to at least
one opening of the first and second end of the tube.
The first middle layer can be of a second polymeric
component optionally comprising one or more metal components which may be a
component described in respect of film, core or inlay.
The medical device with a fabric to be folded to constitute
two polymeric layers as described can have any design and construction and be
of any material as mentioned elsewhere herein.
Bag with a firm shape
In an aspect of the invention a medical device comprises a
bag of a first polymeric component optionally comprising one or more metal
components, wherein the bag has a firm shape in at least one dimension.
In an embodiment the bag has a firm shape in at least two
dimensions. Furthermore the bag may have a firm shape in at least three
dimensions.
In an embodiment the first polymeric component optionally
comprising one or more metal components encircles an inner volume, the inner
volume may be filled with a second polymeric component optionally comprising
one or more metal components before implantation, during implantation and/or
after implantation. The second polymeric component optionally comprising one or
more metal components may be a component described in respect of film, core or
inlay.
In an embodiment the second polymeric component comprises:
fluid polymeric component at least when the polymeric component is filled into
the bag and/or particles of the polymeric component, the particles being
spherical, lentil-shaped, egg- shaped, pyramid-shaped, and/or star-shaped. The
second polymeric component may comprise one or more metal components
In an embodiment the first polymeric component optionally
comprising one or more metal components is a fabric and the fabric is made e.g.
by weaving, knitting and/or crocheting.
The medical device may be manufactured individually
according to the dimensions measured in a joint of a mammal, where the device
is to be inserted.
Medical devices which are soft at the time of implantation
may be inserted into the joint of a mammal by arthroscopy. The device can
continue to be soft within the joint, or harden. The device can also be filled
with another component as described elsewhere herein.
The medical device with a bag with a firm shape can have any
design and construction and be of any material as mentioned elsewhere herein.
Strengthening implants with fabric
In an aspect of the invention a medical device comprises at
least a first surface area, wherein at least a first polymeric component is
attached to the first surface area. The device may at least in the first area
be subjected to wear when the device is located in a mammal individual.
In an embodiment the device is made of polymer, bone and/or
metal. The first polymeric component may constitute at least 1% of the surface
of the device. In an embodiment the first polymeric component optionally
comprising one or more metal components may be located at the outside of the
device in areas that is subjected to wear. The first polymeric component
optionally comprising one or more metal components can be in the form of a
fabric. The fabric can be made by weav- ing, knitting and/or crocheting.
One or more layers of fabric can be attached to the first
surface area. These layers of fabric can be connected by a second polymeric
material optionally comprising one or more metal components e.g. in the form of
a film or core as described elsewhere herein. The fabric may be suitable for
in-growth of cells
In an embodiment the at least first polymeric component
further is placed onto the device in areas that is subjected to a less amount
of wear and/or is not subjected to wear.
Preferred is a hip-joint prosthetic device in which the cup
is fabricated from a multiplicity of layers of fabric. The number of layers can
be between 2 and 100, preferred is between 3 and 50, also preferred is between
4 and 25, more preferred is between 5 and 15. The layers of fabric may be
connected by film or core as mentioned else- where herein. The layers of fabric
may be of different structure in respect of fibre thickness and e.g.
weaving-method, and may also be made of different polymeric materials. The cup
may be manufactured in a composite structure, or individually layers of fabric
may have a composite structure.
The medical device which is strengthened with a fabric and
the fabric used to strengthened the device can have any design and construction
and be of any material as mentioned elsewhere herein.
Finishing treatment of a medical device
The present invention in particular relates to material
formulations intended to meet the specifications of durability,
bio-compatibility, and strength. These properties are obtainable by treating
polymer materials, such as polyethylene, polypropylene or polyvinylpyrrolidone
or combinations and co-polymers thereof as well as precursor materials for
polymerisation, with high-energy electrons, gamma rays, photons, mi- crowaves,
ion implantation, plasma treatment, annealing, thermal radiation or another
radiation to obtain ideal durability and bio-compatibility of the new, modified
material. Treatment of the above-mentioned materials with radiation leads to
cross- linking of polymers and thereby generating new, modified materials.
Preferably, the polymer material is a cross-linked polyethylene or
polypropylene material. More preferably the polymer material is a cross-linked
polyethylene material.
The properties of the materials to be obtained by the
cross-linking process are preferably resistant to tear and wear; and have good
compressibility.
The medical
devicemay be packed in a pouch, which is suitable for irradiation. Preferred
are pouches of aluminium, more preferred are laminated pouches of PE, aluminium
and PET, where PE (polyethylene) comprises the inner of the pouches and PET
(polyethylene-terephthalat) comprises the outer of the pouches.
A pouch with a medical devicemay be filled with nitrogen
before it is made airtight. The medical devices are then subjected to
irradiation to cross-link the polymeric material and sterilise the medical
device.
In order to increase stability of the medical polymeric
device the polymers of the shaped polymer product may be subjected to further
treatment, such as cross linking. In a preferred embodiment the cross linking
treatment is conducted in order to cross link only a fraction of cross linkable
polymers in the product. Accordingly, the products may be cross-linked by
radiation, the cross-linking of the polymers may also be done by other methods
known to the person skilled in the art. The radiation may be but is not limited
to high-energy electrons, gamma rays, photons, and microwaves. Cross binding
the polymers improve the strength of the product. A preferred radiation process
is cross-linking of fibres using treatment with accelerated electrons. As the
cross-linking process takes place in the amorphous polyethylene regions, the
optimal dose will depend on the fraction of amorphous polyethylene in the final
device. The optimal radiation dose is preferably close to the gelation dose of
polyethylene and thus lie between 10-10,000 kGy (0.1 and 100 Mrad), preferred
is between 10 and 300 kGy, most preferred is 200 kGy. The radiation can be
performed in one uninterrupted treatment, where the complete dose of radiation
is given to the material. The radiation process may also be performed in
pulsing or interrupting treatments, where the total dose of radiation is given
in 2-15 shorter with an interval of 1 to 60 minute. Preferred is 25 kGy given
eight times with 10 min interruption between each radiation treatment (total
200 kGy). More preferred are two times 25 kGy interrupted by 1 to 60 minutes
and repeated 4 times with 10 hours to 1 day of interruption.
The
radiation may be performed for the entire product or device or only part of the
product or device is radiated by using a shield or screen between the
irradiation source.
When using radiation, the radiation process described may be
followed by annealing. The purpose of annealing is to eliminate long living
free radicals by a heat treatment of 800C for about 1-12 hours in vacuum. More
preferred is 70-850C for about 16-24 hours in an inert atmosphere. Preferred is
when the inert atmosphere is Nitrogen.
Typically, a device is prepared by a process comprising the
following steps:
• The device is formed under vacuum by pressing the
laminated polymers optionally comprising one or more metal components in a
mould of specified dimensions. The polymer optionally comprising one or more
metal components is chosen from the above mentioned polymers. • After hardening
the material as formed, or after swelling in a suitable solvent, the device may
be subjected to high-energy electrons, gamma rays or another radiation in order
to create cross-linking which will modify the mechanical properties of the
material to meet the preferred specifications.
• Finally, after removal of the swelling solvent, the
surface of the material may be treated to achieve good surface properties as
described elsewhere.
The medical devices may be subjected to annealing when they
are irradiated. Annealing is performed in an oven at about 8O0C for a few hours
to remove residual free radicals. Or annealing is performed as described elsewhere
herein. Surface coating
The surface of the device can subsequently be treated to
modify surface properties such as wetting ability and/or biocompatibility. This
surface treatment can be per- formed by plasma treatment, chemical grafting or
by a combination of plasma polymerisation and chemical grafting. The material
contacting with the biological surfaces may be smooth, biocompatible,
preferably self-lubricating, and it should be wear-resistant so that particles
generated due to wear are avoided in that this could otherwise result in
foreign body reactions and cause further trouble to the function of the part of
organism where the medical device is located.
Furthermore, the surface material should preferably be a
material or a combination of materials having self-repairing properties so that
fissures, cracks or other ruptures on the surface do not exceed uncontrollable
levels. However, the surface material is preferably continuous with the
material of the rest of the device, e.g. the material may gradually merge into
the material of the fabric, film or core of the device. In this context
continuous means that the surface material cannot be pulled away from the
material beneath.
The surface of the material may be chemically treated so as
to soften, rigidify or lubricate the surface of the device or parts thereof.
The surface of the material may be coated so that the coating confers these
properties, or may be treated so as to chemically alter the surface of the
device so as to confer any of these properties. Alternatively, certain polymer
surfaces may be modified by means of thermal or photolytic energy.
Without being bound by theory it is also believed that a
wetted surface reduces the risk of having the immune system recognising the
device when implanted, which would otherwise lead to adverse effects of the
device.
In one embodiment the surface of the devicemay be coated by
a plasma polymerisation, using low-power plasma equipment. The monomers used
for the plasma polymerisation are any monomer forming a hydrophilic polymer by
plasma polymerisation. Preferred are monomers forming polyvinylpyrrolidone and
poly-ethylene- glycol like polymers, most preferred is 1-vinyl-2-pyrrolidinone.
The surface coating performed as described above has a thickness of 1 to 700
nm, such as between 10 and 500 nm, preferable between 20 and 400 nm, more
preferable between 30 and 300 nm, further preferable between 40 and 200 nm, yet
further preferable between 50 and 100 nm, most preferable between 60 and 90 nm.
In another embodiment the surface coating performed as
described above has a thickness of 1 nm to 5,000 nm, such as between 5 and
2,500 nm, preferable between 10 and 1000 nm, more preferable between 30 and 500
nm, further preferable between 40 and 400 nm, yet further preferable between 45
and 300 nm, most preferable between 50 and 250 nm.
Plasma is ionised gas. In an artificial plasma to be used
for plasma treatment and plasma polymerisation, the concentration of ionised
species is preferably 0.1-10 ppm. Two phases exists in artificial plasma: A
gas-phase comprising an energi corresponding to the surrounding temperature,
usually room temperature. In a plasma- phase ions and electrons have an energi
at approximately 2-10 eV.
The artificial plasma may be established by exposing a gas
with electric field. The pressure of the gas is preferably 0.01-1 mbar. The
electric voltage utilised is dependent of different features such as the
pressure, the composition of the gas, electrode configuration, the size of the
polymerisation chamber, and frequencies of the electricity. The voltage is
typically 200-10,00OV.
In a preferred embodiment of the plasma polymerisation
1-vinyl-2-pyrrolidinone (VP) may be polymerised to polyvinylpyrrolidone (PVP)
in a plasma with low energi. The plasma functions as an initiator for the
polymerisation by formation of radicals in the surface of the element to be
coated. From the radicals the polymerisation process takes place where monomers
of VP polymerise to PVP. A low energy is necessary not to destroy the monomer
VP in the gas-phase as well as the polymerised PVP. In a preferred embodiment
the energy is 0.1-1 W/L.
In the plasma polymerisation treatment a carrier gas is
used, preferred is an inert gas, such as argon or helium. The chamber for
performing the plasma treatment is constructed to perform a homogeneous surface
coating of the device by the plasma polymerisation process.
The surface coated polymeric product is preferably
sterilised by radiation or by heating. The radiation can be but is not limited
to high-energy electrons, gamma rays, photons, microwaves.
The polymeric product may by cross-linked and sterilised
simultaneously by treating with ionizing radiation or by heating. Preferred is
cross-linking by radiation.
Mechanical properties
The structure of the material of a device may comprise a
layered or laminated structure, a core of one material or one or more
interposed layers with different properties enabling an overall function of the
devise suitable for providing a spacer function and/or to exert pressure
distribution of joints and/or to provide at least part of the sliding/rotating
movement of joints by internal movement of the device, or relevant part of the
device. However, it is preferred that the material itself does not comprise
interposed layers resulting in sliding between the layers and thereby tear on
the mating surfaces within the device. Accordingly, the body of the device
should be one continuous solid or semi-solid material.
Mechanical properties for certain relevant polymers are
described by Szycher (Szy- cher, M. (editor), sponsored by SPE, Society of
Plastics Engineers, Inc. Biocompati- ble Polymers, Metals, and Composites, pp.
725-727, 757-61).
Mechanical properties of polymers are controlled by the
elastic parameters, the three moduli: elastic, shear, and compressive moduli.
These parameters are theoretically interrelated. A modulus is the ratio between
the applied stress and the cor- responding deformation. The reciprocals of the
moduli are called compliances. The three elastic moduli have the dimension:
force per unit area, (N/m2 or Pa). Polymers are not normally ideal elastic
bodies, but under load they show (time dependant) viscoelastic properties. By
taking the load into consideration, the properties should be viewed according
to this dilemma. Also, ideal elastic properties and ultimate properties are
influenced by the viscoelastic properties. Ultimate tensile strength is a
measure of the stress required to cause the material to rupture in tension.
Ultimate elongation is the percent stretch of the material before it ruptures
in tension. Elongation (%) is measured as
Elongation (percent) =
where SB = observed distance between bench marks of the stretched specimen at rupture, and S0 = the original distance between bench marks.
Table 1 - Elastic parameters and their definitions
Examples of
ranges of the mechanical properties of the device are mentioned below. However,
it should be contemplated that not all of the following characteristics may be
fulfilled by the material of the prosthetic device since, as explained above,
the numerous properties of the material are theoretically interrelated.
Accordingly, conflict in fulfilling all parameters within the stated ranges may
occur.
In one embodiment, the prosthetic device according to the
invention is a device wherein the material of the device or at least the part
of the device which exerts the pressure distribution and/or the part which
exerts the sliding/rotating movement in the joint when the joint is loaded
has/have one or more of the following propeties (under biological conditions
(37°C, physiological salinity)): A compressive modulus (K) of at least 2000
MPa, a shear modulus (G) of at least 1 MPa and an elastic module (E) of at
least 10 MPa.
Furthermore, certain requirements to the material under
stress with forces that ultimately leads to disintegration can be expressed.
Based on the elasticity parameters for the material, the properties of the
material with respect to pressure, elongation, torsion and displacement in the
range where the material responds elastic can be estimated. The ultimate limits
should preferably be within ± 20% of the range of elastic response. As a consequence
thereof, the limits for the ultimate properties (ultimate compression strength,
tensile strength, torsional strength, shearing strength) can be derived.
Furthermore, the material should have an "ultimate percentage
elongation" of at least 20%.
The materials according to the invention may be a
"quasi elastic" material. Y. Shiki- nami and H. Kawarada,
Biomaterials 19, 1998, pp. 617-635, discuss that many materials of biological
origin, has a J-form in a stress vs. strain curve, whereas may synthetic
materials has an S-form.
Preferably, the critical surface tension (γc) values should
be within the "zone of bio- compatibility" corresponding to the range
of about 20-30 dynes/cm (as defined by Lelah M. D., Cooper, S. L.,
Polyurethanes in Medicine- CRC Press, Inc. Boca Raton, Florida, pp. 59-62 and
92-93).
Additives
A device constructed from the polymeric product optionally
comprising one or more metal components may comprise biologically active
additives. Medication or biological active substances can be used as additive
to the device to facilitate healing, minimise destruction or with other
therapeutic goals, such as pain relief, anti- inflammation, oncology
treatments, stimulation of bone growth, and/or anti-infectious agents. Also,
biological osteogenic or chondrogenic, chondral inductive, and/or chondral
conductive materials may be added to the device. In particular patients
suffering from osteoporosis or other bone degenerating conditions may benefit
from having devices comprising osteogenic inductive materials implanted.
The medication or biological active substances can be used
as additive to the device to facilitate cell growth, such as osteocytes,
osteoblasts, chondrocytes, chon- droblasts, mesenchymal cells. Cartilage
inducing factor may for example be the fac- tors described in US 4,774,322 and
US 4,843,063.
In another preferred embodiment, additives such as
lubricants, dyes, stabilizers and other process enhancing compounds are
incorporated into the polymeric mixture. Such compounds may not necessarily
enhance the strength or structural integrity of the final polymeric matrix, but
do aid in the manufacturing process or enhance the overall appearance of the
finished article. Examples of these compounds may be long chain fatty acids and
their salts, organic and inorganic coloring agents, free radical inhibitors, pH
buffering agents and other materials known to enhance proc- essing of polymers
within the polymer industry.
In another preferred embodiment of the present invention,
solid materials may be incorporated into the polymer or resin mixtures. Such
solid materials may be, for example, chopped carbon or glass fiber or
nanotubes, carbon black, graphite pow- der, talc, mica, polyamide fiber and
other fillers commonly used in the polymer industry. As is known in the polymer
industry, such fillers may be advantageously added to a polymer matrix for the
purposes of enhancing strength, durability, bulk density, machineablity of the
resulting polymeric article. Of, course the above list is not exhaustive and
other uses of the fillers may also be contemplated.
Devices
One preferred device produced of the polymeric product
described herein may be a substitution for cartilage. The cartilage
substitution may replace damaged cartilage between intact bones, or it may be
part of a medical prosthesis comprising cartilage substitution.
A device produced of the polymeric product itself can be
used as a growth medium and/or network for the natural or artificial cells,
such as chondrocytes.
A device made from the polymeric product optionally
comprising one or more metal components as described above is capable of being
formed to suit into parts of the organism as described elsewhere herein.
Especially the device is suitable to be used in animals, such as mammals and
human beings, preferred is human beings. The animals, to which the medical
device may be utilised, may be selected from the group of mammals, such as but
not limited to horses, dogs, cats, cows and monkeys. In one embodiment the
device is especially constructed to be utilised to support, hold, sustain,
bear, carry, replace or displace any constitution within the mammalian body,
which comprises high shape stability and good wear resistance.
The polymeric product optionally comprising one or more
metal components is adapted not to interfere with intra-articular or other components
when the device is in the body of a human.
The polymeric product as medical device may be but is not
limited to be used as joint spacer implant in joints of knees, hip, shoulders,
fingers, wrist, elbow, spine, neck, loin, toes and ankles. Especially the
devices may be used in diseased patients with osteoarthritic degeneration of
joints. The implants with a smooth articulating surface oppose the diseased and
degenerated cartilage joint facet, which is expected to lead to reduced force
and stresses and improved mobility in the joint with consequent reduced pain
and improved functional capacity of that joint.
The medical device as described herein may be produced in a
number of sizes corresponding to the natural variety of the bones within the
joint where it is intended to be used as well as to the differences in bone
size due to the age or size of individu- als.
Moreover, non-interference of the intra-articular components
may be achieved by a hole which runs through the body of the device; that is to
say the device may comprise a hole through which intra-articular components may
pass. When loading the device, the slits may serve to pass intra-articular
components through the body of the device. The slits in this embodiment run
from the periphery of the body of the device to the hole through which the
intra-articular components pass after the device is implanted or loaded.
Typically, and to at least some extent, the device is
adapted in its structure and/or material composition to alleviate conditions
associated with worn cartilage by providing a spacer function and/or to exert
pressure distribution in the joint when the joint is loaded and/or to provide
at least part of the sliding/rotating movement of the joint by internal
movement of at least part of the device. It is also an object of the present
invention to provide a method for non-invasive locking of a device within a
joint. In addition, the method is independent of use of cement or bony ingrowth
of the device.
The device may completely or substantially completely
surround an intra-articular component or other components of the organism.
A device made from the polymeric product described above is
capable of being formed to suit any joint cavity of animals or human beings,
therefore the device may for example be formed to fit into any one of the
following joints: Hip joint, knee joint, ankle joints, shoulder joint, elbow
joints, wrist, fingers, spinal column joints, such as for substituting
intervertebral discs, and the jaw joint.
The medical device may constitute the surface of a
prosthetic device. It may be the entire surface or part of the surface of a
prosthetic device Also the device may constitute a complete or part of a hip
endo-prosthesis, or it may be a breast prosthesis, a stent, a catheter, a heart
valve or cartilage substitution.
Generally, the invention comprises the polymeric product
optionally comprising one or more metal components as described above from
which different medical devices may be manufactured, also the method of
producing the polymeric product and medical devices is enclosed within the
invention. Enclosed are methods of producing a polymeric product and medical
devices as described above, as well as any combination of the features
described for the polymeric product and the medical devices.
In another aspect the invention relates to a method for
producing a polymeric product optionally comprising one or more metal
components, the method comprising obtaining a number of at least three polymer
layers optionally comprising one or more metal components, and positioning the
polymer layers in a sandwich composition, forming the sandwich composition of
polymer layers by heating the composition followed by pressing it into a mould,
where the heating and pressing processes are conducted in vacuum, and providing
the polymeric product in a desired shape. In the method for producing a
polymeric product wherein the polymeric product is as described above, at least
three polymer layers may be utilised, these polymer layers may constitute a core
with at least one layer of fabric on each side, where the core differs in
constitution from the fabrics, preferred is the method for producing a
polymeric product where the fabrics at the different sides of the core have
equal constitutions.
The method for producing a polymeric product comprises two
or more layers of fabrics, where the two or more layers of fabrics have a film
of a polymer layer in between each fabric.
In the method for producing a polymeric product the core and
the film have similar composition except for the thickness of the polymer
layer. The thickness of the polymer layers is as described above, in a
preferred embodiment the film is between 0.01 and 2 mm thick, and the core is
between 0.1 and 10 mm thick.
In an embodiment the method for producing a polymeric
product comprises fabric, film and core where the structure of the fabric are
composed of long polymer fibre, and the core and film are composed of short
chain polymers. These polymer fibres can be selected among polyethylene (PE), polypropylene
(PP) and polyvinylpyrrolidone (PVP). Most preferable is polyethylene (PE). The
long polymer fibres are ultra high molecule weight polyethylene (UHMWPE) fibre
and the short chain polymers may be branched.
In an embodiment the method for producing a polymeric
product comprises fabric which is manufactured, e.g., woven, into a shape or
form suitable for the shape of the polymeric product. The fabric consist of
UHMWPE fibres in which the intersects are positioned as formerly described,
preferably in angles of about 90 degree.
In an embodiment the method for producing a polymeric
product comprises fabric which has high tensile strength and high wear
resistance, and a core which absorbs shocks, pushes and strokes.
The method for producing a polymeric product comprises
arranging the polymer layers in the order of fabric, film and core in
accordance to the description above. The most preferred constitutions are
listed above. The polymer layers are heated, and under vacuum the polymeric
product is pressed in to a mould. The device, which is formed, is treated by
ionising radiation, to further cross bind the polymers and thereby improve the
strength of the product. The product is further subjected to annealing to
ensure all linking has appeared.
In an embodiment the method for producing a polymeric
product comprises surface coating of the annealed polymeric product and further
the polymeric product is sterilised by ionising radiation or by heating.
In another embodiment the method for producing a polymeric
product comprises annealing the polymeric product before it is subjected to
surface coating.
In a
further embodiment the method for producing a polymeric product comprises
simultaneously cross-linking and sterilisation of the polymeric product by
treating with ionising radiation or by heating.
In an embodiment the method for producing a polymeric
product comprises surface coating of the polymeric product, as formerly
described.
In a preferred embodiment the method for producing a
polymeric product comprises production of the polymeric product where the shape
and size of the polymeric product can be any possible to produce by pressing
into to a mould, the mould forming a polymeric product which can be flat or
round or in between and where the three-dimensional shape can be any possible
forming by pressing into a mould.
The polymeric product can be utilised to produce a
prosthetic device comprising polymer layers, the order of the polymer layers,
and the method of production of the polymeric product as described above.
Preferred is a method of producing a prosthetic device of
three polymer layers, which constitute a core with at least one layer of fabric
on each side. Another preferred constitution is a core which at each side has
two layers of fabric with a film in between. A further preferred constitution
is a film between two layers of fabric. In a preferable embodiment of the
method the prosthetic device are produced from polymer layers optionally
comprising one or more metal components composed of a polymer selected among
polyethylene (PE), polypropylene (PP) and polyvinylpyrrolidone (PVP). Most
preferable is a prosthetic device wherein the polymer layers are composed of
polyethylene (PE).
In a further preferable embodiment of the method the
prosthetic device are composed of fabrics of long polymer fibre, which
preferable are ultra high molecule weight polyethylene (UHMWPE) fibre or other
polyethylene fibre as previously de- scribed, whereas the core and the film are
composed of short chain polymers, the short chain polymers may be branched.
The fabric is of medical grade and is woven into a shape
suitable for the shape of the polymeric product. The shaping and physical
characteristics is determined by the arrangement of the UHMWPE fibres, the
fibres can have intersects in angles as described formerly.
In a preferred embodiment the prosthetic device has a high
tensile strength and a high wear resistance due to the properties of the
fabrics, whereas the core absorbs shocks, pushes and strokes.
The polymeric constitution of the prosthetic device is
obtained in accordance with the details given above where the polymer layers
are heated, subjected to vacuum and pressed into shape in a mould, and further
treated as described above.
Examples
Example 1
Artificial Cartilage Cup
The artificial cartilage cup is an artificial joint spacer
made to replace the missing or damaged cartilage so the joint can stay mobile.
The cup is based on a sandwich construction with a LDPE core reinforced on both
sides with fibre fabric.
At the edge metal markers makes it possible to trace the cup
when implanted.
The round LDPE collar of the cup makes a cup without sharp
edges and captures the metal markers.
Finally a crosslinking of the polymer improves the
performance of the LDPE core.
The production process may include the following steps:
• Injection moulding of base LDPE disk
The LDPE disk is made of pellets/granulates in the injection
moulding process. The disk is approximately five mm. thick and 134 mm in
diameter. One standard disk size will later be formed to different size of cups.
• Pressure consolidation with fibre fabric
Two pieces of 20X20 cm fibre fabric are placed on each side
of the disk and the sandwich is pressed to form a cup with a surplus material
like an irregular hat brim.
Different cup sizes are produced and identified by an
individual number.
• Shaping the cup by cutting off excess material
Cutting off the hat brim leaves a cup with right angle
edges.
• Drilling of cup holes and mounting of metal markers Metal
markers in the cup make tracing the cup in the body possible. For the first
test production, the markers are tantalum balls, and for later production, the
markers will be stainless steel balls.
• Injection moulding of LDPE-collar on cup
The metal markers are fixed, and LDPE-collar covers the
right angle edges.
• Packaging with nitrogen gas
In a packing machine, the cup is packed in an aluminum pouch
with nitrogen gas, and the pouch is sealed to prevent oxygen from being in
contact with the cup. Oxygen will hinder the later crosslinking process, as it
reacts with the free radicals. The aluminium pouch is put in a shipment box,
ready for sending to the crosslinking plant.
• Crosslinking and Sterilization The cup goes to the
irradiation plant, is irradiated and returns to the production area. The
irradiation forms free radicals. The free radicals are very reactive places in
the polymer material, which react to form crosslinking in the polymer. The
irradiation dose is about 200 kGy.
• Annealing Just after the irradiation process, there are
still free radicals. In the annealing process, the free radicals form
crosslinking. The annealing process is a heating at approximately 75°C, which
speeds up the crosslinking reaction without softening the cup. The process is
slowly running even at room temperature, but might take about one month. The
tem- perature must be at a relatively low level in order to avoid softening and
deforming of the cup.
• Final packing, releasing and storing
The cup is packed in inner box, labelled and instructions
for use are supplied. The product is released after a quality check, and stored
at the subcontractor.
Items
1. A medical device comprising at least a first surface
area, wherein at least a first polymeric component optionally further
comprising one or more metal components is attached to said first surface area.
2. The device according to any of the preceding items,
wherein said first surface area is a part or the entire surface area of a first
volume, where said first volume is smaller than the final volume and where said
final volume is the volume of the device before implantation, and wherein the
difference between said final volume and said first volume is a volume made up
by at least said first polymeric component optionally further comprising one or
more metal components.
3. The device according to any of the preceding items,
wherein said first volume has a shape which corresponds to the final volume of
the device before implantation.
4. The device according to any of the preceding items,
wherein said first vol- ume corresponds to at least 50% of said final volume,
such as at least 60%, such as at least 70%, such as at least 80%, such as at
least 85%, such as at least 90%, such as at least 95%, such as at least 97%,
such as at least 99%.
5. The device according to any of the preceding items,
wherein said first vol- ume corresponds to a lesser degree to said final volume
in areas where said at least first polymeric component optionally further
comprising one or more metal components is attached.
6. The device according to any of the preceding items,
wherein said first vol- ume further comprises different first zones, and where
said different first zones are smaller volumes of said first volume and said
first volume in different first zones corresponds to a different degree to
related final zones of said final volume, and where said final zones are
smaller volumes of said final volume. 7. The device according to any of the
preceding items, wherein the volume of said first zones corresponds to at least
50% of the volume of said related final zones, such as at least 60%, such as at
least 70%, such as at least 80%, such as at least 85%, such as at least 90%,
such as at least 95%, such as at least 97%, such as at least 99%.
8. The device according to any of the preceding items,
wherein the volume of said first zones corresponds to a different degree to the
volume of said related final zones and where said different degree is selected
between 50- 100%, such as 60-100%, such as 70-100%, such as 80-100%, such as
90-
100%, such as 95-100%, such as 50-90%, such as 60-90%, such
as 70- 90%, such as 80-90%, such as 50-80%, such as 60-80%, such as 70-80%,
such as 50-70%, such as 60-70%.
9. The device according to any of the preceding items,
wherein said first volume further correspond to the final shape of said final
volume.
10. The device according to any of the preceding items,
wherein said first surface area comprises the outer surface of one or more of
said first zones.
11. The device according to any of the preceding items,
wherein said at least first polymeric component optionally further comprising
one or more metal components is attached to the entire outer surface of said
first volume.
12. The device according to any of the preceding items,
wherein said device at least in said first area is subjected to wear when said
device is located in an individual.
13. The device according to any of the preceding items,
wherein said first vol- ume of said device is made partly or entirely of
polymer, bone and/or metal.
14. The device according to any of the preceding items,
wherein said first polymeric component optionally further comprising one or
more metal components constitute at least 1 % of the entire outer surface of
the device, such as at least 3%, such as at least 5%, such as at least 8%, such
as at least 10%, such as at least 15%, such as at least 20%, such as at least
25%, such as at least 30%, such as at least 35%, such as at least 40%, such as
at least 50%, such as at least 60%, such as at least 70%, such as at least 80%,
such as at least 90%, such as at least 99%.
15. The device according to any of the preceding items,
wherein said first polymeric component optionally further comprising one or
more metal components constitute at least 1 % of the diameter of said final
volume of the device, such as at least 3%, such as at least 5%, such as at
least 8%, such as at least 10%, such as at least 15%, such as at least 20%,
such as at least
25%, such as at least 30%, such as at least 35%, such as at
least 40%, such as at least 50%, such as at least 60%.
16. The device according to any of the preceding items,
wherein said first poly- meric component optionally further comprising one or
more metal components is located at the outside of said device in areas that is
subjected to wear.
17. The device according to any of the preceding items,
wherein said first poly- meric component optionally further comprising one or
more metal components is in the form of a fabric.
18. The device according to any of the preceding items,
wherein said fabric is made by weaving, knitting and/or crocheting.
19. The device according to any of the preceding items,
wherein said at least first polymeric component optionally further comprising
one or more metal components further is placed onto said device in areas that
is subjected to a lesser amount of wear than in said first surface area and/or
is not subjected to wear.
20. The device according to any of the preceding items,
wherein said device further comprises at least a second polymeric component
optionally further comprising one or more metal components which is attached at
least to said first surface area, and wherein the chain length of the first
polymeric component is longer than the chain length of the second polymeric
component.
21. A medical device according to any of the preceding
items, wherein said device has an upper surface, a lower surface and at least
one edge and wherein at least said one edge is sealed by a collar.
22. The device according to any of the preceding items,
wherein said collar is of said first, second and/or a third polymeric component
optionally further comprising one or more metal components.
23. The device according to any of the preceding items,
wherein at least one of said first, second or third polymeric component
optionally further comprising one or more metal components of said collar is a
X-ray sensitive polymeric component.
24. The device according to any of the preceding items,
wherein said collar is sealed to said first and/or said second polymeric
component optionally fur- ther comprising one or more metal components of the device.
25. The device according to any of the preceding items,
wherein said collar covers at least 0.5 mm of said upper surface when measured
from said edge.
26. The device according to any of the preceding items,
wherein said collar covers at least 0.5 mm of said lower surface when measured
from said edge.
27. The device according to any of the preceding items,
wherein said device further comprises at least one marker.
28. The device according to any of the preceding items,
wherein said at least one marker is one or more materials which are different
from the implant materials in a way such that these materials can be used as a
marker in MR, X ray and/or PET investigations. 29. The device according to any
of the preceding items, wherein said at lerast one marker is selected from the
group of metals or contrast medium.
30. The device according to any of the preceding items,
wherein said at least one marker is at least a thread of metal.
31. The device according to any of the preceding items,
wherein said at least one marker is supported by and/or incorporated into said
collar.
32. The device according to any of the preceding items,
wherein said at least one marker is located at the edge of said device and said
collar encloses said marker.
33. The device according to any of the preceding items,
wherein said at least one marker is a thread of gold.
34. The device according to any of the preceding items,
wherein said at least one marker is in the form of at least two units, and said
two units is placed non-homogeneous within said device.
35. A medical device any of the preceding items, comprising
at least a first and a second side, wherein said at least first side is made of
a first polymeric component optionally further comprising one or more metal
components and wherein said first and/or second side has a frictional resistance
of less than 0.5 Newton.
36. The device according to any of the preceding items,
wherein said first polymeric component optionally further comprising one or
more metal components has a frictional resistance of less than 0.5 Newton and
said first poly- meric component optionally further comprising one or more
metal components further is located in at least a first area where said device
is subjected to wear when said device has been implanted into an individual.
37. The device according to any of the preceding items, wherein said wear in
said at least first area is due to friction made by a bone and/or a medical
device.
38. The device according any of any of the preceding items,
wherein said second side is made of a first and/or a second polymeric component
optionally further comprising one or more metal components.
39. The device according to any of the preceding items,
wherein said first and/or second side further includes at least one cavity.
40. The device according to any of the preceding items,
wherein said at least one cavity has a diameter of at least 0.05 mm when
measured at the outside of said first side.
41. The device according to any of the preceding items,
wherein said at least one cavity has a depth of at least 0.01 mm.
42. A medical device according to any of the preceding
items, wherein said de- vice has at least one through-going perforation which
is not for ligaments.
43. The device according to any of the preceding items,
wherein said at least one perforation has a diameter of at least 0.01 mm.
44. The device according to any of the preceding items,
wherein said at least one perforation is located in at least one area where
said device is subjected to wear.
45. The device according to any of the preceding items,
wherein said at least one perforation is located homogeneous over substantially
the entire device.
46. The device according to any of the preceding items,
wherein said at least one perforation can conduct liquid from a first side of
said device to a second side of said device and/or from said second side to
said first side. 47. The device according to any of the preceding items,
wherein said liquid is synovial fluid or physiological salt solution or another
biocompatible liquid.
48. The device according to any of the preceding items, wherein
said device fur- ther comprises an inner volume between said first and second
side and where said liquid can be within.
49. The device according to any of the preceding items,
wherein said inner volume further comprises a network of at least one polymeric
and/or metal com- ponent.
50. The device according to any of the preceding items,
wherein said at least one polymeric and/or metal component is polymeric fibres
and/or metal fibres.
51. The device according to any of the preceding items,
wherein said inner volume comprises a storage of liquid.
52. A medical device according to any of the preceding
items, wherein said first polymeric component optionally further comprising one
or more metal components is self-healing when subjected to injury before implantation,
during implantation and/or after implantation.
53. The device according to any of the preceding items,
wherein said polymeric component optionally further comprising one or more
metal components is a composite polymeric component optionally further
comprising one or more metal components.
54. The device according to any of the preceding items,
wherein said polymeric component optionally further comprising one or more
metal components includes a microencapsulated healing agent that is released upon
injuries, and wherein polymerization of the polymeric component is triggered by
contact with an embedded catalyst/initiator. 55. The device according to any of
the preceding items, wherein said healing agent is monomers of said first
polymeric component and/or of a second polymeric component.
56. A medical device according to any of the preceding
items, comprising at least a first unit with at least a convex surface and a
second unit with at least a concave surface, where said convex and concave
surface is congruent with each other and said first unit fit partially or
entirely into said second unit, and wherein said first and/or second unit
comprises at least one polymeric component optionally further comprising one or
more metal components.
57. The device according to any of the preceding items,
wherein said first and second unit have substantially similar size.
58. The device according to any of the preceding items,
wherein said first and second unit have different sizes.
59. The device according to any of the preceding items,
wherein said first and second units are connected or substantially connected by
said convex and concave surface.
60. The device according to any of the preceding items,
wherein said concave surface is at least 0.01 mm smaller than said convex
surface.
61. The device according to any of the preceding items,
wherein said units are movable compared to each other, and said units continue
to be in contact during a movement and/or return to be in contact when a
movement is finished.
62. The device according to any of the preceding items,
wherein said first unit and/or said second unit is attached to an implant
and/or a bone.
63. The device according to any of the preceding items,
wherein said first unit and/or said second unit can function without being
attached to an implant and/or a bone. 64. A medical device according to any of
the preceding items, comprising at least an upper layer, a first middle layer
and a lower layer, wherein said upper layer and said lower layer is made from
one uninterrupted piece of at least a first polymeric component optionally
further comprising one or more metal components.
65. The device according to any of the preceding items,
wherein said piece of at least a first polymeric component optionally further
comprising one or more metal components is in the form of a fabric.
66. The device according to any of the preceding items,
wherein said piece of at least a first polymeric component optionally further
comprising one or more metal components is constructed in one piece, which is folded
around an axis and hereby forming said upper and lower layer.
67. The device according to any of the preceding items,
wherein said upper and lower layer are similar or substantially similar in
size.
68. The device according to any of the preceding items,
wherein said at least a first polymeric component optionally further comprising
one or more metal components is in the form of fibres.
69. The device according to any of the preceding items,
wherein said piece of at least a first polymeric component optionally further
comprising one or more metal components is in the form of a tube before folded
around said axis.
70. The device according to any of the preceding items,
wherein said tube has a uniform diameter along said tube.
71. The device according to any of the preceding items,
wherein said tube contract in a first and/or a second end of said tube.
72. The device according to any of the preceding items,
wherein said tube con- tract in said axis. 73. The device according to any of
the preceding items, wherein said axis further comprises at least one aperture.
74. The device according to any of the preceding items,
wherein said fabric and said tube is made by weaving, knitting or crocheting.
75. The device according to any of the preceding items,
wherein said axis is located substantially at the middle of said tube and said
axis is parallel or sub- stantial parallel to at least one opening of said
first and second end of said tube.
76. A medical device according to any of the preceding
items, comprising at least a first polymeric component optionally further
comprising one or more metal components, wherein said device has a middle area
which at least in one dimension is surrounded by an outer area, and wherein the
thickness of said outer area at least partly is larger than the thickness of
said middle area, and said outer area ends in at least one edge of said device.
77. The device according to any of the preceding items,
wherein said middle area is surrounded by said outer area in two dimensions.
78. The device according to any of the preceding items,
wherein said device is homogenous in dimensions around an axis, and wherein
said axis is a cen- tral axis according to one dimension of the middle area.
79. The device according to any of the preceding items,
wherein said device is heterogeneous in dimensions around an axis, and wherein
said axis is an approximately central axis according to one dimension of the
middle area.
80. The device according to any of the preceding items,
wherein said middle area and said at least one edge are in different planes.
81. The device according to any of the preceding items,
wherein when said at least one edge are in different planes, and these planes
are projected to a similar plane, this constitute an outline of said at least
one edge, and said outline has a shape that is selected from a shape from
triangular to circular.
82. The device according to any of the preceding items,
wherein said device is cup-shaped or approximately cup-shaped, and wherein said
middle area is a top and said outer area is a skirt.
83. The device according to any of the preceding items,
wherein the device is a cup-shaped device with a rounded top, a line separating
said top and said skirt is equator, and the outmost part of the skirt is the
edge of the device.
84. The device according to any of the preceding items,
wherein the thickness of said outer area is at least 5% larger than the
thickness of said middle area.
85. The device according to any of the preceding items,
wherein said device is part of a hip joint prosthesis, and where said device
comprises a spacer e.g. a cup shaped spacer which is positioned between the
natural femoral stem or a metal femoral stem and a natural acetabular cup or a
prosthetic acetabular cup.
86. A medical device according to any of the preceding
items, comprising a 3D network of polymer fibre and/or metal fibre.
87. The device according to any of the preceding items,
wherein an area de- fined by outermost fibres of said network is filled with
one or more polymer optionally further comprising one or more metal components
or said area is filled with a metal or metal alloy.
88. The device according to any of the preceding items,
wherein said device is an acetabular cup, a spacer to be located between an
acetabular cup and a head of hip stem, or an interpositional arthroplastry.
89. The device according to any of the preceding items,
wherein said polymeric components optionally further comprising one or more
metal components are in layers of said device. 90. The device according to any
of the preceding items, wherein said layered structure comprises
• at least one upper layer of said first polymeric component
optionally further comprising one or more metal components,
• a middle layer of said second polymeric component
optionally further comprising one or more metal components, and
• at least one lower layer of said third polymeric component
optionally further comprising one or more metal components, wherein the chain length
of the first polymeric component and the third polymeric component is longer
than the chain length of the second polymeric component.
91. The device according to any of the preceding items,
wherein at least one upper layer and the at least one lower layer each are
composed of at least two layers of polymeric fabric optionally further
comprising one or more metal components constructed of said first and third
polymeric components, and at least one layer of polymeric film optionally
further comprising one or more metal components, said polymeric film
constitutes a layer between two layers of said polymeric fabrics.
92. The device according to any of the preceding items,
wherein the polymeric components optionally further comprising one or more
metal components of the middle layer and the polymeric components optionally
further comprising one or more metal components of the film are substantially
identical.
93. The device according to any of the preceding items,
wherein said one or more metal components are selected from the group of metal
and metal alloys of titanium, gold, silver, chromium-cobaltum, zirconia,
cobalt- chromium-molobdenum alloy and Stainless Steel alloys and/or a ceramic
of one or more of these metals and alloys.
94. The device according to any of the preceding items,
wherein said one or more metal components are in the a powder, granulate,
chopped fibres, long fibres, 2D structural components like plates, 3D
structural components like shaped plates or hemicircles with holes. Also a
combination of these forms may be used.
95. The device according to any of the preceding items,
wherein the first poly- meric component has a carbon-backbone.
96. The device according to any of the preceding items,
wherein the first polymeric component optionally further comprising one or more
metal components and the second polymeric component optionally further
comprising one or more metal components are compounded to form a bidispergent
system.
97. The device according to any of the preceding items,
wherein the first polymeric component is selected from polyacrylates,
polystyrene, polyethers, polytetrafluorethylene, polyvinylalcohol,
polyethylene, polyethylene oxides, polyvinylpyrrolidon and polypropylene.
98. The device according to any of the preceding items, wherein
the first polymeric component is a composite material.
99. The device according to any of the preceding items,
wherein the first polymeric component is nanofibre.
100. The device according to any of the preceding items,
wherein the second polymeric component is selected from polyacrylates,
polystyrene, poly- ethers, polytetrafluorethylene, polyvinylalcohol,
polyethylene, polyethylene oxides, polyvinylpyrrolidon and polypropylene.
101. The device according to any of the preceding items,
wherein the second polymeric component is a composite material.
102. The device according to any of the preceding items,
wherein the second polymeric component is nanofibre.
103. The device according to any of the preceding items,
wherein the sec- ond polymeric component is cross-linked. 104. The device
according to any of the preceding items, wherein the first and the second
polymeric component comprises the same monomeric component or the same
components of the composite material.
105. The device according to any of the preceding items,
wherein the first and the second polymeric component comprises the same
monomeric component or the same components of the composite material and where
the first and second polymeric components are differently crystallized within
the implant ready to implant.
106. The device according to any of the preceding items,
wherein the first and the second polymeric component comprises the same
monomeric component or the same components of the composite material and where
the first and second polymeric components have different morphology within the
implant ready to implant.
107. The device according to any of the preceding items,
further comprising a third polymeric component optionally further comprising
one or more metal components which is attached at least to said first surface
area, said third polymeric component being different from the first and/or the
second polymeric component.
108. The device according to any of the preceding items,
wherein the third polymeric component is grafted to the first and/or the second
polymeric components.
109. The device according to any of the preceding items,
wherein the chain length of the first polymeric component is above 100 monomer
units.
110. The device according to any of the preceding items,
wherein the first polymeric component comprises a copolymer of polyethylene and
polypropylene, and the second polymer is grafted to the first polymer. 111. The
device according to any of the preceding items, wherein the first polymeric
component is a cross-linked polymer, and the second polymer is grafted to the
first polymer.
112. The device according to any of the preceding items,
wherein the first polymeric component is a composite polymer, and the second
polymer is grafted to the first polymer.
113. The device according to any of the preceding items,
wherein the first and/or second polymer component is obtained by cross-linking
polyethylene, polypropylene or polyvinylpyrrolidone or combinations or
co-polymers thereof.
114. The device according to any of the preceding items,
wherein the cross- linking is achieved with radiation.
115. The device according to any of the preceding items,
wherein the forms of radiation are selected from the group comprising
high-energy electrons, gamma rays, photons, microwaves, and thermal radiation.
116. The device according to any of the preceding items,
wherein at least one of said polymeric components are subjected or further
subjected to surface treatment to obtain optimised wetting ability and to
obtain biocompatibil- ity and resistance to heat treatment for sterilisation.
117. The device according to any of the preceding items,
wherein at least one of the polymeric components comprises polypropylene,
preferably cross- linked polypropylene.
118. The device according to any of the preceding items,
wherein the first polymeric component and the third polymeric component is
above 100 monomer units, such as above 1000 monomers units, for example above
10000 monomer units, preferable above 20000 monomer units, more preferable
above 30000 monomer units, further preferable above 40000 monomer units, yet
further preferable above 50000 monomer units, most preferable above 60000
monomer units.
119. The device according to any of the preceding items,
wherein the third polymeric component is selected from polyacrylates,
polystyrene, polyethers, polytetrafluorethylene, polyvinylalcohol,
polyethylene, polypropylene, polyethylene oxides and polyvinylpyrrolidon.
120. The device according to any of the preceding items,
wherein the first, second and/or third polymeric component is a composite
material.
121. The device according to any of the preceding items,
wherein the first, second and/or third polymeric component is nanofibre.
122. The device according to any of the preceding items,
wherein the first polymeric component and the third polymeric component are
substantially identical.
123. The device according to any of the preceding items,
wherein the polymeric components comprises a copolymer of polyethylene and/or
polypropylene, preferable of polyethylene (PE).
124. The device according to any of the preceding items,
wherein the first and third polymeric components are composed of long polymer
fibre, and the second polymeric component is a short chain polymer material.
125. The device according to any of the preceding items,
wherein the first and third polymeric components are ultra high molecule weight
polyethylene (UHMWPE) fibre.
126. The device according to any of the preceding items,
wherein said first polymeric component optionally further comprising one or
more metal components is in the form of a fabric. 127. The device according to
any of the preceding items, wherein said second polymeric component optionally
further comprising one or more metal components is in the form of a fabric.
128. The device according to any of the preceding items,
wherein said third polymeric component optionally further comprising one or
more metal components is in the form of a fabric.
129. The device according to any of the preceding items,
wherein said fabric is made of polymer and/or metal fibre with similar
thickness.
130. The device according to any of the preceding items,
wherein said fabric is made of polymer and/or metal fibre with different
thickness.
131. The device according to any of the preceding items,
wherein the fabric is produced in a shape suitable for the shape of the medical
product
132. The device according to any of the preceding items,
wherein said shape of the fabric is constructed by weave, knit, crochet, plait,
interlace, intertwine, interlock, link or unite the fibre in other ways,
preferable the fabric is woven or knitted.
133. The device according to any of the preceding items,
wherein the polymer and/or metal fibres in the fabric are crossing each other
in intersects which are positioned in angles of 1 to 179 degree, such as in
angles of 80 to
100 degree, preferable in angles of about 90 degree.
134. The device according to any of the preceding items,
wherein the thickness of at least one fabric in the device varies across the
device.
135. The device according to any of the preceding items,
wherein the thickness of said at least one fabric has a first thickness in the
middle area of said device and a second thickness in at least a part of the
outer area of said device. 136. The device according to any of the preceding
items, wherein said first thickness is smaller than said second thickness.
137. The device according to any of the preceding items,
wherein said first thickness is larger than said second thickness.
138. The device according to any of the preceding items,
wherein the first, second and third polymeric components optionally further
comprising one or more metal components are of medical grade.
139. The device according to any of the preceding items,
wherein the fabric has a high tensile strength and a high wear resistance.
140. The device according to any of the preceding items,
wherein the tensile strength of a fibre or strand of the fabric is abovei .O
GPa, such as above 1.2 Gpa, preferable above 1.4 Gpa, more preferable above 1.6
Gpa, further preferable above 1.8 Gpa, yet further preferable above 1.9 Gpa,
most preferable above 2.0 Gpa,
141. The device according to any of the preceding items,
wherein the polymers of the second polymeric component are short chain
polymeric material which may be branched.
142. The device according to any of the preceding items,
wherein the film is between 0.001 and 5 mm thick, such as between 0.01 and 5
mm, preferable between 0.1 and 4 mm, more preferable between 0.2 and 3 mm,
further preferable between 0.3 and 2 mm, yet further preferable between 0.4 and
1.5 mm, most preferable between 0.5 and 1 mm.
143. The device according to any of the preceding items, wherein
the middle layer may be a core, a film or an inlay.
144. The device according to any of the preceding items,
wherein the core is between 0.1 and 30 mm thick, such as between 0.2 and 25 mm,
preferable between 0.3 and 21 mm, more preferable between 0.4 and 17 mm,
further preferable between 0.5 and 13 mm, yet further preferable between 0.6
and 10 mm, most preferable between 0.7 and 7 mm.
145. The device according to any of the preceding items,
wherein said film and/or core is made of varying length of fibres, nano-fibre,
chopped fibres, composite material, composite material including nano-fibre,
nano-structured composite, armoured polymers and/or varying length of fibres
and/or varying length of nano-fibres.
146. The device according to any of the preceding items,
wherein the constitution of the polymeric product is: fabric - film - fabric.
147. The device according to any of the preceding items,
wherein the constitution of the polymeric product is: fabric - core - fabric.
148. The device according to any of the preceding items,
wherein a number of film and fabric are positioned at one or both sides of the
mentioned polymeric product in a way where film and fabric alternate in the
polymeric product.
149. The device according to any of the preceding items,
wherein the number of fabric and film in said upper layer and in said lower
layer is not equal.
150. The device according to any of the preceding items,
wherein the number of core, film, inlay and fabrics is varied in different
areas of the device.
151. The device according to any of the preceding items,
wherein the number of fabric is between 1 and 100, such as between 2 and 50,
for example between 2 and 40, preferable between 2 and 35, more preferable
between 2 and 30, further preferable between 2 and 25, yet further preferable
between 2 and 20, most preferable between 2 and 10. 152. The device according
to any of the preceding items, wherein the number of film is between 0 and 100,
such as between 1 and 50, for example between 1 and 40, preferable between 1
and 35, more preferable between 1 and 30, further preferable between 1 and 25,
yet further preferable between 1 and 20, most preferable between 1 and 10.
153. The device according to any of the preceding items,
wherein the number of core is between 0 and 100, such as between 1 and 50, for
example between 1 and 40, preferable between 1 and 35, more preferable between
1 and 30, further preferable between 1 and 25, yet further preferable between 1
and 20, most preferable between 1 and 10.
154. The device according to any of the preceding items,
wherein the number of inlays is between 0 and 50, such as between 1 and 40, for
example between 1 and 30, preferable between 1 and 25, more preferable between
1 and 20, further preferable between 1 and 15, yet further preferable between 1
and 10, most preferable between 1 and 5.
155. The device according to any of the preceding items,
wherein the polymeric layers optionally further comprising one or more metal
components in areas or in the entire of some layers has a constitution where
film and core or film and inlay are placed towards each other.
156. The device according to any of the preceding items,
wherein said first polymeric component optionally further comprising one or
more metal components and/or said second polymeric component optionally further
comprising one or more metal components and/or said third polymeric component
optionally further comprising one or more metal components are attached to said
first volume by ultrasound welding, laser welding, heating and/or gluing.
157. The device according to any of the preceding items,
wherein said first polymeric component optionally further comprising one or
more metal components and/or said second polymeric component optionally further
comprising one or more metal components and/or said third polymeric component
optionally further comprising one or more metal components are attached to each
other before attached to said first volume.
158. The device according to any of the preceding items,
wherein said first polymeric component optionally further comprising one or
more metal components and/or said second polymeric component optionally further
comprising one or more metal components and/or said third polymeric optionally
further comprising one or more metal components component are attached to said
first volume one polymeric component at a time.
159. The device according to any of the preceding items,
wherein the number of layers of said polymeric components includes more than
one layer of one of the polymeric components optionally further comprising one
or more metal components, and each of said layers are attached to said first
volume one polymeric component at a time.
160. The device according to any of the preceding items,
wherein the device further comprises a hole extending through the body of the
device.
161. The device according to any of the preceding items,
wherein the device further comprises a slit in the body of the device extending
through the body of the device from the surface of the body to the hole.
162. The device according to any of the preceding items,
wherein the de- vice further comprises a means of enabling a passage through
the body of the device to the hole.
163. The device according to any of the preceding items,
wherein said device is adapted to alleviate conditions associated with worn
cartilage by pro- viding a spacer function and/or to exert pressure
distribution in the joint when the joint is loaded and/or to provide at least
part of the sliding/rotating movement of the joint by internal movement of at
least part of the device.
164. The device according to any of the preceding items,
wherein the de- vice is capable of locking itself to an intra-articular
component and thereby being fixed or retained in the joint cavity in a manner
which is substantially non-invasive with respect to cartilage and bone natively
present in the joint cavity.
165. The device according to any of the preceding items,
wherein the polymer material meets mechanical properties in that the E modulus
(Young's modulus) is at least 10 MPa.
166. The prosthetic according to any of the preceding items,
wherein the device comprises more than one more unit.
167. The device according to any of the preceding items,
wherein the units are adapted not to interfere with intra-articular components
when the device is in the joint cavity.
168. The device according to any of the preceding items,
wherein the body of the unit further comprises a hole extending through the
body of the device.
169. The device according to any of the preceding items,
wherein the body of the unit further comprises a slit extending from the
surface of the body to the hole.
170. The device according to any of the preceding items,
wherein the surface treatment results in a material with critical surface
tension (γc) values within the "zone of biocompatibility" of 20-30
dynes/cm.
171. The device according to any of the preceding items,
which is capable of locking itself to the intra-articular component by at least
one element of the device surrounding the component in such a manner that
displacement of the element is limited by interlocking with said component.
172. The device according to any of the preceding items,
wherein the element completely or substantially completely surrounds an
intra-articular component being a ligament. 173. The device according to any of
the preceding items, which device, when present in situ, comprises at least one
ring-shaped element.
174. The device according to any of the preceding items for
the articulation of a joint of a mammal.
175. The device according to any of the preceding items,
wherein said joint is selected from hip joint, knee joint, ankle joint,
shoulder joint, elbow joint, wrist, fingers, feet, toes, jaw-joint, midfoot,
talus-calcaneus, spinal column joints, such as for substituting intervertebral
discs, and the jaw joint.
176. The device according to any of the preceding items,
wherein said mammal is selected from humans, horses, dogs, cats, cows and
cattle, elephants, swine, and monkeys.
177. The device according to any of the preceding items,
wherein said device is a hip endoprothese.
178. The device according to any of the preceding items,
wherein said de- vice comprises a metal or ceramic femoral stem articulating
against an PE acetabular cup, a UHMWPE acetabular cup or a metal acetabular
cup, where said femoral stem and/or said acetabular cup are covered according
to any of the preceding items.
179. The device according to any of the preceding items,
having such shape and/or properties that it is capable of replacing or
supplementing worn or damaged cartilage in the joint and/or is capable of
preventing wear of the native cartilage of the joint.
180. The device according to any of the preceding items,
wherein the diameter of the device in situ and when the joint is loaded is such
that it substantially covers the surface area of the load bearing part of the
joint which in the normal joint is covered with cartilage.
181. The
device according to any of the preceding items, wherein the joint is the hip
joint, and wherein the diameter of the device is such that the surface of caput
femoris is substantially covered when the joint is loaded.
182. The device according to any of the preceding items,
wherein diameter of the device is between 1-80 mm, such as between 2-70 mm,
preferable between 10-60 mm, more preferable between 15-50 mm, when the joint
is loaded.
183. The device according to any of the preceding items,
wherein the thickness of the device in the middle area is between 0.2-60 mm,
such as between 0.3-40 mm, preferable 0.6-30 mm, more preferable about 0.8-20
mm, most preferable about 1-15 mm in the unloaded stage.
184. The device according to any of the preceding items,
wherein the thickness of the device in the outer area and closest to the edge
is between 0.2- 60 mm, such as between 0.3-40 mm, preferable 0.6-30 mm, more
preferable about 0.8-20 mm, most preferable about 1-15 mm in the unloaded
stage.
185. The device according to any of the preceding items,
wherein the device comprises parts overlapping each other.
186. The device according to any of the preceding items,
wherein the overlapping parts, on their mating surfaces have an interlocking
surface struc- ture.
187. The device according to any of the preceding items,
wherein the interlocking surface structures constitute grooves.
188. The device according to any of the preceding items,
wherein the interlocking surface structures are grooved in a radial direction.
189. The device according to any of the preceding items,
wherein the interlocking surface structures are grooved in a circular
direction. 190. The device according to any of the preceding items, wherein the
interlocking surface structures constitute elevations and corresponding
depressions.
191. The device according to any of the preceding items,
wherein the E modulus (Young's modulus) of the material of at least part of the
device is at least 10 MPa, such as at least 13 MPa, preferably at least 16 MPa,
more preferable at least 19 MPa, still more preferable at least 22 MPa, most preferable
at least 25 MPa, such as at least 30 MPa or 50 MPa.
192. The device according to any of the preceding items,
wherein the polymer components optionally further comprising one or more metal
components are connected by heating to a temperature between 80 and 250 degree
Celsius, such as between 90 and 240 degree Celsius, preferable between 100 and
230 degree Celsius, more preferable between 110 and 220 degree Celsius, further
preferable between 120 and 210 degree Celsius, yet further preferable between
130 and 200 degree Celsius, most preferable between 140 and 190 degree Celsius.
193. The device according to any of the preceding items,
wherein the polymer components optionally further comprising one or more metal
components in the connecting process further are subjected to vacuum, such as a
vacuum below 500 mbar, preferable below 300 mbar, more preferable below 100
mbar, further preferable below 50 mbar, yet further preferable below 10 mbar,
most preferable below 1 mbar.
194. The device according to any of the preceding items,
wherein when heated the polymers of the core or the film or the inlay penetrate
into the fibres of the fabrics, and hereby mechanically connect the polymer
layers to each other.
195. The device according to any of the preceding items,
wherein the temperature is selected to a level where the fibre of the fabrics
are not melted. 196. The device according to any of the preceding items,
wherein the temperature is selected to a level where the main part of the
fabrics is not melted, but a thin layer constituting a low number of fibres of
the outer part of the outermost fabrics of the polymeric product is melted.
197. The device according to any of the preceding items,
wherein the shape of the device is any shape which can be formed by pressing
into a mould, said shape can constitute a surface which may be but is not
limited to flat, curved, waved, undulated, bent, bowed, crooked, while the
overall shape of the device may be but is not limited to circular, oval,
squared, rectangle, cubed, bowl, cup, crown, cap, basin, preferred shape is cup
or hemispherical.
198. The device according to any of the preceding items,
wherein to the polymeric material optionally further comprising one or more
metal components is attached to a component, said component being polymeric or
non-polymeric.
199. The device according to any of the preceding items,
wherein the device is supplied with one or more apertures, holes, gaps,
perforations or hollows.
200. The device according to any of the preceding items, wherein
said device is utilised to support, bear, carry, replace or displace any
constitution within the human body, which comprises high shape stability and
good wear resistance.
201. The device according to any of the preceding items,
wherein at least one of said layers has dimensions which extend at least
approximately over the entire device and towards said at least one edge.
202. The device according to any of the preceding items,
wherein said at least one edge is heat treated. 203. The device according to
any of the preceding items, wherein a collar is attached to said at least one
edge.
204. The device according to any of the preceding items,
wherein said col- lar is moulded before attached to said device.
205. The device according to any of the preceding items,
wherein said collar further includes and/or support at least one marker.
206. The device according to any of the preceding items,
wherein said collar further includes polymer and/or metal fibre.
207. The device according to any of the preceding items,
wherein said fibre is any polymeric fibre, e.g. UHMWPE, nanofibre, short fibre.
208. The device according to any of the preceding items,
wherein said collar is secured to said device by ultrasound welding, gluing,
sewing and/or laser welding.
209. The device according to any of the preceding items,
wherein at least part of said polymeric component or polymeric components of
said device, is suitable for cells to grow into it.
Claims:
1. A medical device comprising
at least one fabric of one or more polymer fibre and/or of one or more metal
fibre and/or a 3D network of polymer fibre and/or metal fibre.
2. The device according to any
of the preceding claims, wherein a volume at least defined by the outermost
fibres of said network is filled up with one or more polymer optionally further
comprising one or more metal components or said volume is filled with a metal
or metal alloy.
3. The device according to any
of the preceding claims, wherein said volume at least defined by the outermost
fibres of said network is a volume defined by the final device and said network
of fibres is larger than said volume defined by the final device.
4. The device according to any
of the preceding claims, wherein said polymer fibre and/or said polymer optionally
further comprising one or more metal components is a polyolefin, such as
polyethylene, such as UHMWPE.
5. The device according to any
of the preceding claims, wherein said metal fibre and/or said metal or metal
alloy is selected from the group of titanium, gold, silver, chromium-cobaltum,
zirconia, cobalt-chromium-molobdenum alloy and Stainless Steel alloys and/or a
ceramic of one or more of these metals and alloys
6. The device according to any
of the preceding claims, wherein said device is an acetabular cup, a spacer to
be located between an acetabular cup and a head of hip stem, or an
interpositional arthroplastry.
7. A medical device according
to any of the preceding claims comprising at least a first surface area,
wherein at least a first polymeric component optionally further comprising one
or more metal components is attached to said first surface area.
8. The device according to any
of the preceding claims, wherein said first surface area is a part or the
entire surface area of a first volume, where said first volume is smaller than
the final volume and where said final volume is the volume of the device before
implantation, and wherein the difference be- tween said final volume and said
first volume is a volume made up by at least said first polymeric component
optionally further comprising one or more metal components.
9. The device according to any
of the preceding claims, wherein said first volume has a shape which
corresponds to the final volume of the device before implantation.
10. The device according to
any of the preceding claims, wherein said first volume corresponds to at least
50% of said final volume, such as at least 60%, such as at least 70%, such as
at least 80%, such as at least 85%, such as at least 90%, such as at least 95%,
such as at least 97%, such as at least 99%.
11. The device according to
any of the preceding claims, wherein said first volume corresponds to a lesser
degree to said final volume in areas where said at least first polymeric
component optionally further comprising one or more metal components is
attached.
12. The device according to
any of the preceding claims, wherein said first volume further comprises
different first zones, and where said different first zones are smaller volumes
of said first volume and said first volume in different first zones corresponds
to a different degree to related final zones of said final volume, and where
said final zones are smaller volumes of said final volume.
13. The device according to
any of the preceding claims, wherein the volume of said first zones corresponds
to at least 50% of the volume of said related final zones, such as at least
60%, such as at least 70%, such as at least 80%, such as at least 85%, such as
at least 90%, such as at least 95%, such as at least 97%, such as at least 99%.
14. The device according to
any of the preceding claims, wherein the volume of said first zones corresponds
to a different degree to the volume of said related final zones and where said
different degree is selected between 50- 100%, such as 60-100%, such as
70-100%, such as 80-100%, such as 90- 100%, such as 95-100%, such as 50-90%,
such as 60-90%, such as 70-
90%, such as 80-90%, such as
50-80%, such as 60-80%, such as 70-80%, such as 50-70%, such as 60-70%.
15. The device according to
any of the preceding claims, wherein said first vol- ume further correspond to
the final shape of said final volume.
16. The device according to
any of the preceding claims, wherein said first surface area comprises the
outer surface of one or more of said first zones.
17. The device according to
any of the preceding claims, wherein said at least first polymeric component
optionally further comprising one or more metal components is attached to the
entire outer surface of said first volume.
18. The device according to
any of the preceding claims, wherein said device at least in said first area is
subjected to wear when said device is located in an individual.
19. The device according to
any of the preceding claims, wherein said first volume of said device is made
partly or entirely of polymer, bone and/or metal.
20. The device according to
any of the preceding claims, wherein said first polymeric component optionally
further comprising one or more metal components constitute at least 1% of the
entire outer surface of the device, such as at least 3%, such as at least 5%,
such as at least 8%, such as at least 10%, such as at least 15%, such as at
least 20%, such as at least 25%, such as at least 30%, such as at least 35%,
such as at least 40%, such as at least 50%, such as at least 60%, such as at
least 70%, such as at least 80%, such as at least 90%, such as at least 99%.
21. The device according to
any of the preceding claims, wherein said first polymeric component optionally
further comprising one or more metal components constitute at least 1 % of the
diameter of said final volume of the device, such as at least 3%, such as at
least 5%, such as at least 8%, such as at least 10%, such as at least 15%, such
as at least 20%, such as at least
25%, such as at least 30%,
such as at least 35%, such as at least 40%, such as at least 50%, such as at
least 60%.
22. The device according to
any of the preceding claims, wherein said first polymeric component optionally
further comprising one or more metal components is located at the outside of
said device in areas that is subjected to wear.
23. The device according to
any of the preceding claims, wherein said first polymeric component optionally
further comprising one or more metal components is in the form of a fabric.
24. The device according to
any of the preceding claims, wherein said fabric is made by weaving, knitting
and/or crocheting.
25. The device according to
any of the preceding claims, wherein said at least first polymeric component
optionally further comprising one or more metal components further is placed
onto said device in areas that is subjected to a lesser amount of wear than in
said first surface area and/or is not subjected to wear.
26. The device according to
any of the preceding claims, wherein said device further comprises at least a
second polymeric component optionally further comprising one or more metal
components which is attached at least to said first surface area, and wherein
the chain length of the first polymeric component is longer than the chain
length of the second polymeric component.
27. A medical device according
to any of the preceding claims, wherein said device has an upper surface, a
lower surface and at least one edge and wherein at least said one edge is sealed
by a collar.
28. The device according to
any of the preceding claims, wherein said collar is of said first, second
and/or a third polymeric component optionally further comprising one or more
metal components.
29. The device according to
any of the preceding claims, wherein at least one of said first, second or
third polymeric component optionally further comprising one or more metal
components of said collar is a X-ray sensitive polymeric component.
30. The device according to
any of the preceding claims, wherein said collar is sealed to said first and/or
said second polymeric component optionally further comprising one or more metal
components of the device.
31. The device according to
any of the preceding claims, wherein said collar covers at least 0.5 mm of said
upper surface when measured from said edge.
32. The device according to
any of the preceding claims, wherein said collar covers at least 0.5 mm of said
lower surface when measured from said edge.
33. The device according to
any of the preceding claims, wherein said device further comprises at least one
marker.
34. The device according to
any of the preceding claims, wherein said at least one marker is one or more
materials which are different from the implant materials in a way such that these
materials can be used as a marker in MR, X ray and/or PET investigations.
35. The device according to
any of the preceding claims, wherein said at lerast one marker is selected from
the group of metals or contrast medium.
36. The device according to
any of the preceding claims, wherein said at least one marker is at least a
thread of metal.
37. The device according to
any of the preceding claims, wherein said at least one marker is supported by
and/or incorporated into said collar.
38. The device according to
any of the preceding claims, wherein said at least one marker is located at the
edge of said device and said collar encloses said marker.
39. The device according to
any of the preceding claims, wherein said at least one marker is a thread of
gold.
40. The device according to
any of the preceding claims, wherein said at least one marker is in the form of
at least two units, and said two units is placed non-homogeneous within said
device.
41. A medical device any of
the preceding claims, comprising at least a first and a second side, wherein
said at least first side is made of a first polymeric component optionally
further comprising one or more metal components and wherein said first and/or
second side has a frictional resistance of less than 0.5 Newton.
42. The device according to
any of the preceding claims, wherein said first poly- meric component
optionally further comprising one or more metal components has a frictional
resistance of less than 0.5 Newton and said first polymeric component optionally
further comprising one or more metal components further is located in at least
a first area where said device is subjected to wear when said device has been
implanted into an individual.
43. The device according to
any of the preceding claims, wherein said wear in said at least first area is
due to friction made by a bone and/or a medical device.
44. The device according any
of any of the preceding claims, wherein said second side is made of a first
and/or a second polymeric component optionally further comprising one or more
metal components.
45. The device according to
any of the preceding claims, wherein said first and/or second side further
includes at least one cavity.
46. The device according to
any of the preceding claims, wherein said at least one cavity has a diameter of
at least 0.05 mm when measured at the outside of said first side.
47. The device according to
any of the preceding claims, wherein said at least one cavity has a depth of at
least 0.01 mm.
48. A medical device according
to any of the preceding claims, wherein said device has at least one
through-going perforation which is not for ligaments.
49. The device according to
any of the preceding claims, wherein said at least one perforation has a
diameter of at least 0.01 mm.
50. The device according to
any of the preceding claims, wherein said at least one perforation is located
in at least one area where said device is subjected to wear.
51. The device according to
any of the preceding claims, wherein said at least one perforation is located
homogeneous over substantially the entire device.
52. The device according to
any of the preceding claims, wherein said at least one perforation can conduct
liquid from a first side of said device to a second side of said device and/or
from said second side to said first side.
53. The device according to
any of the preceding claims, wherein said liquid is synovial fluid or
physiological salt solution or another biocompatible liquid.
54. The device according to
any of the preceding claims, wherein said device further comprises an inner
volume between said first and second side and where said liquid can be within.
55. The device according to
any of the preceding claims, wherein said inner volume further comprises a
network of at least one polymeric and/or metal component.
56. The device according to
any of the preceding claims, wherein said at least one polymeric and/or metal
component is polymeric fibres and/or metal fibres.
57. The device according to
any of the preceding claims, wherein said inner volume comprises a storage of
liquid.
58. A medical device according
to any of the preceding claims, wherein said first polymeric component
optionally further comprising one or more metal components is self-healing when
subjected to injury before implantation, during implantation and/or after
implantation.
59. The device according to
any of the preceding claims, wherein said polymeric component optionally
further comprising one or more metal components is a composite polymeric
component optionally further comprising one or more metal components.
60. The device according to
any of the preceding claims, wherein said polymeric component optionally
further comprising one or more metal components includes a microencapsulated
healing agent that is released upon injuries, and wherein polymerization of the
polymeric component is triggered by contact with an embedded
catalyst/initiator.
61. The device according to
any of the preceding claims, wherein said healing agent is monomers of said
first polymeric component and/or of a second polymeric component.
62. A medical device according
to any of the preceding claims, comprising at least a first unit with at least
a convex surface and a second unit with at least a concave surface, where said
convex and concave surface is congruent with each other and said first unit fit
partially or entirely into said second unit, and wherein said first and/or
second unit comprises at least one polymeric component optionally further
comprising one or more metal components.
63. The device according to
any of the preceding claims, wherein said first and second unit have
substantially similar size.
64. The device according to
any of the preceding claims, wherein said first and second unit have different
sizes.
65. The device according to
any of the preceding claims, wherein said first and second units are connected
or substantially connected by said convex and concave surface.
66. The device according to
any of the preceding claims, wherein said concave surface is at least 0.01 mm
smaller than said convex surface.
67. The device according to
any of the preceding claims, wherein said units are movable compared to each
other, and said units continue to be in contact during a movement and/or return
to be in contact when a movement is finished.
68. The device according to
any of the preceding claims, wherein said first unit and/or said second unit is
attached to an implant and/or a bone.
69. The device according to
any of the preceding claims, wherein said first unit and/or said second unit can
function without being attached to an implant and/or a bone.
70. A medical device according
to any of the preceding claims, comprising at least an upper layer, a first
middle layer and a lower layer, wherein said up- per layer and said lower layer
is made from one uninterrupted piece of at least a first polymeric component
optionally further comprising one or more metal components.
71. The device according to
any of the preceding claims, wherein said piece of at least a first polymeric
component optionally further comprising one or more metal components is in the
form of a fabric.
72. The device according to
any of the preceding claims, wherein said piece of at least a first polymeric
component optionally further comprising one or more metal components is constructed
in one piece, which is folded around an axis and hereby forming said upper and
lower layer.
73. The device according to
any of the preceding claims, wherein said upper and lower layer are similar or
substantially similar in size.
74. The device according to
any of the preceding claims, wherein said at least a first polymeric component
optionally further comprising one or more metal components is in the form of
fibres.
75. The device according to
any of the preceding claims, wherein said piece of at least a first polymeric
component optionally further comprising one or more metal components is in the
form of a tube before folded around said axis.
76. The device according to
any of the preceding claims, wherein said tube has a uniform diameter along
said tube.
77. The device according to
any of the preceding claims, wherein said tube contract in a first and/or a
second end of said tube.
78. The device according to
any of the preceding claims, wherein said tube contract in said axis.
79. The device according to
any of the preceding claims, wherein said axis further comprises at least one
aperture.
80. The device according to
any of the preceding claims, wherein said fabric and said tube is made by
weaving, knitting or crocheting.
81. The device according to
any of the preceding claims, wherein said axis is located substantially at the
middle of said tube and said axis is parallel or substantial parallel to at
least one opening of said first and second end of said tube.
82. A medical device according
to any of the preceding claims, comprising at least a first polymeric component
optionally further comprising one or more metal components, wherein said device
has a middle area which at least in one dimension is surrounded by an outer area,
and wherein the thickness of said outer area at least partly is larger than the
thickness of said middle area, and said outer area ends in at least one edge of
said device.
83. The device according to
any of the preceding claims, wherein said middle area is surrounded by said
outer area in two dimensions.
84. The device according to
any of the preceding claims, wherein said device is homogenous in dimensions
around an axis, and wherein said axis is a central axis according to one
dimension of the middle area.
85. The device according to
any of the preceding claims, wherein said device is heterogeneous in dimensions
around an axis, and wherein said axis is an approximately central axis
according to one dimension of the middle area.
86. The device according to
any of the preceding claims, wherein said middle area and said at least one
edge are in different planes.
87. The device according to
any of the preceding claims, wherein when said at least one edge are in
different planes, and these planes are projected to a similar plane, this
constitute an outline of said at least one edge, and said outline has a shape
that is selected from a shape from triangular to circular.
88. The device according to
any of the preceding claims, wherein said device is cup-shaped or approximately
cup-shaped, and wherein said middle area is a top and said outer area is a
skirt.
89. The device according to
any of the preceding claims, wherein the device is a cup-shaped device with a
rounded top, a line separating said top and said skirt is equator, and the
outmost part of the skirt is the edge of the device.
90. The device according to
any of the preceding claims, wherein the thickness of said outer area is at
least 5% larger than the thickness of said middle area.
91. The device according to
any of the preceding claims, wherein said device is part of a hip joint
prosthesis, and where said device comprises a spacer e.g. a cup shaped spacer
which is positioned between the natural femoral stem or a metal femoral stem
and a natural acetabular cup or a prosthetic acetabular cup.
92. The device according to
any of the preceding claims, wherein said polymeric components optionally
further comprising one or more metal components are in layers of said device.
93. The device according to
any of the preceding claims, wherein said layered structure comprises
• at least one upper layer of
said first polymeric component optionally further comprising one or more metal
components,
• a middle layer of said
second polymeric component optionally further comprising one or more metal
components, and
• at least one lower layer of
said third polymeric component optionally further comprising one or more metal
components, wherein the chain length of the first polymeric component and the
third polymeric component is longer than the chain length of the second
polymeric component.
94. The device according to
any of the preceding claims, wherein at least one upper layer and the at least
one lower layer each are composed of at least two layers of polymeric fabric
optionally further comprising one or more metal components constructed of said
first and third polymeric components, and at least one layer of polymeric film
optionally further comprising one or more metal components, said polymeric film
constitutes a layer between two layers of said polymeric fabrics.
95. The device according to
any of the preceding claims, wherein the polymeric components optionally
further comprising one or more metal components of the middle layer and the
polymeric components optionally further comprising one or more metal components
of the film are substantially identical.
96. The device according to
any of the preceding claims, wherein said one or more metal components are
selected from the group of metal and metal alloys of titanium, gold, silver,
chromium-cobaltum, zirconia, cobalt- chromium-molobdenum alloy and Stainless
Steel alloys and/or a ceramic of one or more of these metals and alloys.
97. The device according to any of the preceding claims, wherein said one or more metal components are in the powder, granulate, chopped fibres, long fibres, 2D structural components like plates, 3D structural components like shaped plates or hemicircles with holes. Also a combination of these forms may be used.
External links
Sporring SL, Olsen O, Lauritzen JB, Andersen TL,
Brondsted P, Bechgaard K, Hansen JG, Jensen H, Almdal K, Mouritsen S. Medical
device for insertion into a joint. WO2006133711A2 July
14, 2005. 2006. patents.google
Publications
of invention
EP1896088 (A2)
US2009234459 (A1)
WO2006133711 (A2)
WO2006133711 (A3)
2006SporringSL_MouritsenS
Authors & Affiliations
Sune Lund
Sporring, Naestved (DK)
Ole Olsen,
Charlottenlund (DK)
Jes Bruun
Lauritzen, Kobenhavn K (DK)
Tom Logstrup Andersen,
Roskilde (DK)
Povl Brondsted,
Koge (DK);
Klaus Bechgaard,
Kobenhavn O (DK)
Jan Guldberg
Hansen, Greve (DK)
Henrik Jensen,
Vanlose (DK)
Kristoffer
Almdal, Roskilde (DE)
Soren Mouritsen,
Birkerod (DK)
Keywords
ligamentum capitis femoris, ligamentum teres, ligament
of head of femur, endoprosthesis, prosthesis,
invention, unipolar, subtotal
NB! Fair practice / use: copied for the purposes of criticism, review, comment, research and private study in accordance with Copyright Laws of the US: 17 U.S.C. §107; Copyright Law of the EU: Dir. 2001/29/EC, art.5/3a,d; Copyright Law of the RU: ГК РФ ст.1274/1.1-2,7








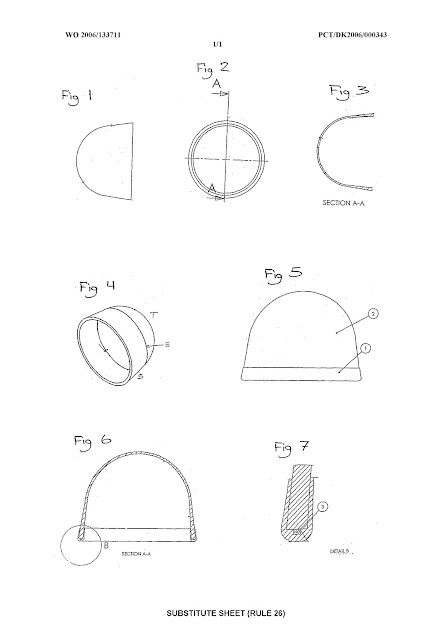
Comments
Post a Comment