Invention (Patent Application Publication): Lang P,
Steines D, Bouadi H, Miller D. Minimally invasive joint implant with
3-dimensional geometry matching the articular surfaces. US20040133276A1 (2004).
Inventors: Philipp Lang, Daniel Steines, Hacene Bouadi, David Miller, Barry Linder, Cecily Snyder
Current Assignee: Conformis Inc
Application US10/681,750 events:
First worldwide family litigation filed
2003-10-07 Application filed by Imaging Therapeutics Inc
2003-10-07 Priority to US10/681,750
2004-07-08 Publication of US20040133276A1
Status: Abandoned
Minimally invasive joint
implant with 3-dimensional geometry matching the articular surfaces.
Philipp Lang, Daniel
Steines, Hacene Bouadi, David Miller, Barry Linder, Cecily Snyder
Abstract
This
invention is directed to orthopedic implants and systems. The invention also
relates to methods of implant design, manufacture, modeling and implantation as
well as to surgical tools and kits used therewith. The implants are designed by
analyzing the articular surface to be corrected and creating a device with an
anatomic or near anatomic fit; or selecting a pre-designed implant having
characteristics that give the implant the best fit to the existing defect.
Description
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001]
This application
claims priority to U.S. Provisional Patent Application 60/416,601
filed by Philipp Lang on Oct. 7, 2002 for “Minimally Invasive Joint Implant
with 3-Dimensional Geometry Matching the Articular Surfaces” and
U.S. Provisional Patent Application 60/467,686 filed by Philipp Lang,
Daniel Steines, Hacene Bouadi, David Miller, Barry J. Linder, and Cecily Anne
Snyder for “Joint Implants” on May 2, 2003.
FIELD OF THE
INVENTION
[0002]
This invention is
directed to orthopedic implants and systems. The implants can be joint implants
and/or interpositional joint implants. The invention also relates to methods of
implant design, manufacture, modeling and implantation as well as to surgical
tools and kits used therewith. This invention also relates to a self-expandable
orthopedic implant amendable to arthroscopic insertion and profile alteration.
Finally, this invention is related to joint implants that are shaped such that
the implants re-establish normal, or near normal, 3D articular geometry or
alignment and facilitate joint movement that exceeds from 60 to 99.9% of the
normal range of motion for the joint and which are capable of withstanding up
to 100% of the normal shear force exerted on the joint during motion.
BACKGROUND OF THE
INVENTION
[0003]
There are various
types of cartilage, e.g., hyaline cartilage and fibrocartilage. Hyaline
cartilage is found at the articular surfaces of bones, e.g., in the joints, and
is responsible for providing the smooth gliding motion characteristic of
moveable joints. Articular cartilage is firmly attached to the underlying bones
and measures typically less than 5 mm in thickness in human joints, with
considerable variation depending on the joint and more particularly the site
within the joint. In addition, articular cartilage is aneural, avascular, and
alymphatic. In adult humans, this cartilage derives its nutrition by a double
diffusion system through the synovial membrane and through the dense matrix of
the cartilage to reach the chondrocyte, the cells that are found in the
connective tissue of cartilage.
[0004]
Adult cartilage has
a limited ability of repair; thus, damage to cartilage produced by disease,
such as rheumatoid arthritis and/or osteoarthritis, or trauma can lead to
serious physical deformity and debilitation. Furthermore, as human articular
cartilage ages, its tensile properties change. Thus, the tensile stiffness and
strength of adult cartilage decreases markedly over time as a result of the
aging process.
[0005]
For example, the
superficial zone of the knee articular cartilage exhibits an increase in
tensile strength up to the third decade of life, after which it decreases
markedly with age as detectable damage to type II collagen occurs at the
articular surface. The deep zone cartilage also exhibits a progressive decrease
in tensile strength with increasing age, although collagen content does not
appear to decrease. These observations indicate that there are changes in
mechanical and, hence, structural organization of cartilage with aging that, if
sufficiently developed, can predispose cartilage to traumatic damage.
[0006]
Usually, severe
damage or loss of cartilage is treated by replacement of the joint with a
prosthetic material, for example, silicone, e.g. for cosmetic repairs, or
suitable metal alloys. See, e.g., U.S. Pat. No. 6,383,228 to Schmotzer, issued
May 7, 2002; U.S. Pat. No. 6,203,576 to Afriat, et al., issued Mar. 20, 2001;
U.S. Pat. No. 6,126,690 to Ateshian et al., issued Oct. 3, 2000. Implantation
of these prosthetic devices is usually associated with loss of underlying
tissue and bone without recovery of the full function allowed by the original
cartilage and, with some devices, serious long-term complications associated
with the loss of significant amount of tissue and bone can include infection,
osteolysis and also loosening of the implant.
[0007]
As can be
appreciated, joint arthroplasties are highly invasive and require surgical
resection of the entire, or a majority of the, articular surface of one or more
bones involved in the repair. Typically with these procedures, the marrow space
is fairly extensively reamed in order to fit the stem of the prosthesis within
the bone. Reaming results in a loss of the patient's bone stock and over time
osteolysis will frequently lead to loosening of the prosthesis. Further, the
area where the implant and the bone mate degrades over time requiring the
prosthesis to eventually be replaced. Since the patient's bone stock is
limited, the number of possible replacement surgeries is also limited for joint
arthroplasty. In short, over the course of 15 to 20 years, and in some cases
even shorter time periods, the patient can run out of therapeutic options
ultimately resulting in a painful, non-functional joint.
[0008]
The use of matrices,
tissue scaffolds or other carriers implanted with cells (e.g., chondrocyte,
chondrocyte progenitors, stromal cells, mesenchymal stem cells, etc.) has also
been described as a potential treatment for cartilage repair. See, also,
International Publications WO 99/51719 to Fofonoff published Oct. 14, 1999; WO
01/91672 to Simon et al., published Dec. 6, 2001; and WO 01/17463 to Mansmann,
published Mar. 15, 2001; and U.S. Pat. No. 6,283,980 B1 to Vibe-Hansen, et al.,
issued Sep. 4, 2001; U.S. Pat. No. 5,842,477 to Naughton, et al., issued Dec.
1, 1998; U.S. Pat. No. 5,769,899 to Schwartz, issued Jun. 23, 1998; U.S. Pat.
No. 4,609,551 to Caplan et al., issued Sep. 2, 1986; U.S. Pat. No. 5,041,138 to
Vacanti et al., issued Aug. 20, 1991; U.S. Pat. No. 5,197,985 to Caplan et al.,
issued Mar. 30, 1993; U.S. Pat. No. 5,226,914 to Caplan, et al., issued Jul.
13, 1993; U.S. Pat. No. 6,328,765 to Hardwick et al., issued Dec. 11, 2001;
U.S. Pat. No. 6,281,195 to Rueger et al., issued Aug. 28, 2001; and U.S. Pat.
No. 4,846,835 to Grande, issued Jul. 11, 1989. However, clinical outcomes with
biologic replacement materials such as allograft and autograft systems and
tissue scaffolds have been uncertain since most of these materials cannot
achieve a morphologic arrangement or structure similar to or identical to that
of the normal, disease-free human tissue it is intended to replace. Moreover,
the mechanical durability of these biologic replacement materials remains
uncertain.
[0009]
U.S. Pat. No.
6,206,927 to Fell, et al., issued Mar. 21, 2001, and U.S. Pat. No. 6,558,421 to
Fell, et al., issued May 6, 2003, disclose a surgically implantable knee
prosthesis that does not require bone resection. This prosthesis is described
as substantially elliptical in shape with one or more straight edges. Accordingly,
these devices are not designed to substantially conform to the actual shape
(contour) of the remaining cartilage in vivo and/or the underlying bone. Thus,
integration of the implant can be extremely difficult due to differences in
thickness and curvature between the patient's surrounding cartilage and/or the
underlying subchondral bone and the prosthesis.
[0010]
Thus, there remains
a need for a system and method for replicating the natural geography of a joint
using one or more implant parts that can be implanted using minimally invasive
techniques and tools for making those repairs and implants and methods that
recreate natural or near natural three-dimensional geometric relationships
between two articular surfaces of the joint.
SUMMARY OF THE
INVENTION
[0011]
The present
invention provides methods and compositions for repairing joints, particularly
devices and implants useful for repairing articular cartilage and for
facilitating the integration of a wide variety of cartilage and bone repair
materials into a subject. Among other things, the techniques described herein
allow for the production of devices that substantially or completely conform to
the contour of a particular subject's underlying cartilage and/or bone and/or
other articular structures. In addition, the devices also preferably
substantially or completely conform to the shape (size) of the cartilage. When
the shape (e.g., size, thickness and/or curvature) of the articular cartilage
surface is an anatomic or near anatomic fit with the non-damaged cartilage,
with the subject's original cartilage, and/or with the underlying bone, the
success of repair is enhanced.
[0012]
The repair material
can be shaped prior to implantation and such shaping can be based, for example,
on electronic images that provide information regarding curvature or thickness
of any “normal” cartilage surrounding a defect or area of diseased cartilage
and/or on curvature of the bone underlying or surrounding the defect or area of
diseased cartilage, as well as bone and/or cartilage comprising the opposing
mating surface for the joint.
[0013]
The current
invention provides, among other things, for minimally invasive methods for
partial joint replacement. The methods can result in little or no loss in bone
stock resulting from the procedure. Additionally, the methods described herein
help to restore the integrity of the articular surface by achieving an anatomic
or near anatomic fit between the implant and the surrounding or adjacent
cartilage and/or subchondral bone.
[0014]
In most cases, joint
mobility for the repaired joint will range from 60 to 99.9% of normal mobility.
The range of motion is improved to 85-99.9%, more preferably between 90-99.9%,
most preferably between 95-99.9% and ideally between 98-99.9%.
[0015]
Further, the incisions
required to implant the devices of the invention typically are less than 50% of
the incision required to implant currently available implants. For example, a
total knee replacement typically employs an incision of from 6-12 inches (15-30
cm) while a unicompartmental knee replacement requires an incision of 3 inches
(7 cm). An implant according to this invention designed to repair the tibial
surface requires only a 3 cm incision (approximately 1.5 inches), while a
combination of implants for repairing both the tibial surface and the femoral
condyles requires an incision of 3 inches (7 cm). In another example, a
traditional hip replacement surgery requires a single incision of between 6 and
12 inches (15-30 cm), or in the less invasive technique two incisions of 1.5-4
inches (3-9.5 cm). An implant according to this invention designed to repair
the acetabulum requires a single incision of from 1.5 inches (3 cm) to 6 inches
(30 cm), depending upon whether single or dual surface correction is desired.
[0016]
Advantages of the
present invention can include, but are not limited to, (i) customization of
joint repair to an individual patient (e.g. patient specific design or
solution), thereby enhancing the efficacy and comfort level following the
repair procedure; (ii) eliminating the need for a surgeon to measure the defect
to be repaired intraoperatively in some embodiments; (iii) eliminating the need
for a surgeon to shape the material during the implantation procedure; (iv)
providing methods of evaluating curvature or shape of the repair material based
on bone, cartilage or tissue images or based on intraoperative probing
techniques; (v) providing methods of repairing joints with only minimal or, in
some instances, no loss in bone stock; and (vi) improving postoperative joint
congruity.
[0017]
Thus, the design and
use of joint repair material that more precisely fits the defect (e.g., site of
implantation) and, accordingly, provides improved repair of the joint is
described herein.
[0018]
As can be
appreciated by those of skill in the art an implant is described that is an
interpositional articular implant, cartilage defect conforming implant,
cartilage projected implant, and/or subchondral bone conforming implant. The
implant has a superior surface and an inferior surface. The superior surface
opposes a first articular surface of a joint and the inferior surface opposes a
second articular surface of the joint and further wherein at least one of the
superior or inferior surfaces has a three-dimensional shape that substantially
matches the shape of one of the first and second articular surfaces. The
implant is suitable for placement within any joint, including the knee, hip,
shoulder, elbow, wrist, finger, toe, and ankle. The superior surface and the
inferior surface of the implant typically have a three dimensional shape that
substantially matches the shape of at least one of the articular surface that
the superior surface of the implant abuts and the inferior surface of the
articular surface that the implant abuts. The implant is designed to have a
thickness of the cartilage defect in a patient, or a fraction thereof,
typically between 65% and 99.9%.
[0019]
The implant can be
manufactured from a variety of suitable materials, including biocompatible
materials, metals, metal alloys, biologically active materials, polymers, and
the like. Additionally, the implant can be manufactured from a plurality of
materials, including coatings.
[0020]
The implant can
further have a mechanism for attachment to a joint. Suitable attachment
mechanisms include ridges, pegs, pins, cross-members, teeth and protrusions.
Additional mechanisms for stabilization of the joint can be provided such as
ridges, lips, and thickening along all or a portion of a peripheral surface.
[0021]
The implant can also
be designed such that it has two or more components. These components can be
integrally formed, indivisibly formed, interconnectedly formed, and
interdependently formed, depending on the desired functionality. In the
multiple component scenario, the joint contacting components can be designed to
engage the joint slideably or rotatably, or a combination thereof.
Alternatively, either or both of the joint contacting components can be fixed
to the joint. Any additional components can be integrally formed, indivisibly
formed, interconnectedly formed or interdependently formed with any other
component that it engages.
[0022]
Each component of
the implant, or the implant itself can have a shape formed along its periphery
or perimeter that is circular, elliptical, ovoid, kidney shaped, substantially
circular, substantially elliptical, substantially ovoid, and substantially
kidney shaped. Additionally, each component of the implant, or the implant
itself can have a cross-sectional shape that is spherical, hemispherical,
aspherical, convex, concave, substantially convex, and substantially concave.
[0023]
The design of the
implant is such that it is conducive for implantation using an incision of 10
cm or less. Further, the implant is designed to restore the range of motion of
the joint to between 80-99.9% of normal joint motion.
[0024]
The implant, or any
component thereof, can have a variety of shapes such that the periphery of the
implant can be of greater thickness than a central portion of the implant. Alternatively,
the implant, or any component thereof, can be designed so that the central
portion of the implant is of greater thickness than a periphery. Looking at the
implant from a plurality of directions, such as an anterior portion, posterior
portion, lateral portion and medial portion, the implant, or any component
thereof, can have a thickness along the posterior portion of the device that is
equal to or greater than a thickness of at least one of the lateral, medial and
anterior portion of the implant. Alternatively, the implant, or any component
thereof, can have a thickness along a posterior portion of the device that is
equal to or less than a thickness of at least one of the lateral, medial and
anterior portion of the implant. In yet another alternative, the implant, or
any component thereof, can have a thickness along a medial portion of the
device that is equal to or less than a thickness of at least one of an anterior
portion, posterior portion, and lateral portion. In another alternative, the implant
can have a thickness along a medial portion of the device that is equal to or
greater than a thickness of at least one of an anterior portion, posterior
portion, and lateral portion.
[0025]
Procedures for
repairing a joint using the implant described below includes the step of
arthroscopically implanting an implant having a superior and inferior surface
wherein at least one of the superior or inferior surfaces has a
three-dimensional shape that substantially matches the shape of an articular
surface. The image can be analyzed prior to implantation. Typically the image
is an MRI, CT, x-ray, or a combinations thereof.
[0026]
The method of making
an implant according to this invention includes: determining three-dimensional
shapes of one or more articular surface of the joint; and producing an implant
having a superior surface and an inferior surface, wherein the superior surface
and inferior surface oppose a first and second articular surface of the joint
and further wherein at least one of the superior or inferior surfaces
substantially matches the three-dimensional shape of the articular surface.
[0027]
Further, the present
invention provides novel devices and methods for replacing a portion (e.g.,
diseased area and/or area slightly larger than the diseased area) of a joint
(e.g., cartilage and/or bone) with an implant material, where the implant
achieves an anatomic or near anatomic fit with at least one surface of the
surrounding structures and tissues and restores joint mobility to between
60-99.9% of the normal range of motion for the joint. Further, the implants can
withstand up to 100% of the shear force exerted on the joint during motion. In
cases where the devices and/or methods include an element associated with the
underlying articular bone, the invention also provides that the bone-associated
element can achieve an anatomic or near anatomic alignment with the subchondral
bone. The invention also enables the preparation of an implantation site with a
single cut. These devices can be interpositional. The devices can be single
component, dual component, or have a plurality of components.
[0028]
A method of the
invention comprises the steps of (a) measuring one or more dimensions (e.g.,
thickness and/or curvature and/or size) of the intended implantation site or
the dimensions of the area surrounding the intended implantation site; and (b)
providing cartilage replacement or material that conforms to the measurements
obtained in step (a). In certain aspects, step (a) comprises measuring the
thickness of the cartilage surrounding the intended implantation site and
measuring the curvature of the cartilage surrounding the intended implantation
site. Alternatively, step (a) can comprise measuring the size of the intended
implantation site and measuring the curvature of the cartilage surrounding the
intended implantation site; or measuring the thickness of the cartilage
surrounding the intended implantation site, measuring the size of the intended
implantation site, and measuring the curvature of the cartilage surrounding the
intended implantation site; or reconstructing the shape of healthy cartilage
surface at the intended implantation site; or measuring the size of the
intended implantation site and/or measuring the curvature or geometry of the
subchondral bone at the or surrounding the intended implantation site. In
addition, the thickness, curvature or surface geometry of the remaining
cartilage at the implantation site can be measured and can, for example, be
compared with the thickness, curvature or surface geometry of the surrounding
cartilage. This comparison can be used to derive the shape of a cartilage
replacement or material more accurately.
[0029]
The dimensions of
the replacement material can be selected following intraoperative measurements,
for example measurements made using imaging techniques such as ultrasound, MRI,
CT scan, x-ray imaging obtained with x-ray dye and fluoroscopic imaging. A
mechanical probe (with or without imaging capabilities) can also be used to
selected dimensions, for example an ultrasound probe, a laser, an optical
probe, an indentation probe, and a deformable material.
[0030]
One or more
implantable device(s) includes a three-dimensional body. In a knee, the implant
can be used in one (unicompartmental) or more (multicompartmental)
compartments. In the knee, the implant is not elliptical in shape, but follows
the 3D geometry of the articular cartilage, subchondral bone and/or
intra-articular structures. The implant has a pair of opposed faces. The
contours of one face of the implant matches or substantially match the
underlying cartilage and/or bone contour; while the contour of the opposing
face of the implant creates a surface for a mating joint surface to interface
with. For example, the surface of the opposing face can be projected using
modeling to optimize the surface for mating with the joint. In addition, the
opposed faces can be connected using a rounded interface. The interface can
also extend beyond the articular surface. The implants of the invention can
also be self-expandable and amendable to arthroscopic insertion.
[0031]
Each face of the
device is not necessarily uniform in dimension. The length D across one axis
taken at any given point is variable along that axis. Similarly the length 2D
across the second axis (perpendicular to the first axis) is also variable along
that axis as well. The ratio between any D length along a first axis and any D
length along a second axis can have any ratio that is suitable for the physical
anatomy being corrected and would be appreciated by those of skill in the art.
[0032]
As will be
appreciated by those of skill in the art, any of the implantable joint
prostheses described herein can comprise multiple (e.g., two or more pieces)
body components that are engageable (e.g., slideably) and/or separable without
departing from the scope of the invention. For example, a two-piece component
can be provided where each component has a face whose contour conforms,
partially or substantially, to the underlying cartilage and/or bone. In certain
embodiments, the opposing surfaces of the components that are engageable are
curved. The curvature can be selected to be similar to that or mirror that of
at least one articular surface for that joint. In other embodiments, the
opposing surfaces of the components that are engageable are flat. In other
embodiments, the opposing surfaces of the components that are engageable are a
combination of flat and curved. The opposing surfaces of the components that
are engageable can also be irregular. In this case, they are preferably
designed to mate with each other in at least one or more positions.
[0033]
In any of the
methods described herein, the replacement material can be selected (for
example, from a pre-existing library of repair systems). Thus, the replacement
material can be produced pre-, intra- or post-operatively. Furthermore, in any
of the methods described herein the replacement material can also be shaped
using appropriate techniques known in the art; either pre-operatively,
intra-operatively, or post-operatively. Techniques include: manually,
automatically or by machine; using mechanical abrasion including polishing,
laser ablation, radiofrequency ablation, extrusion, injection, molding, compression
molding and/or machining techniques, or the like. Finally, the implants can
comprise one or more biologically active materials such as drug(s), cells,
acellular material, pharmacological agents, biological agents, and the like.
[0034]
The invention
includes a method of repairing cartilage in a subject, the method comprising
the step of implanting cartilage repair material prepared according to any of
the methods described herein. Implantation is typically arthroscopic and can be
accomplished via a relatively small incision.
[0035]
The invention also
provides a method of determining the curvature of an articular surface, the
method comprising the step of intraoperatively measuring the curvature of the
articular surface using a mechanical probe or a surgical mechanical navigation
system. The articular surface can comprise cartilage and/or subchondral bone.
The mechanical probe (with or without imaging capabilities) can include, for
example an ultrasound probe, a laser, a mechanical arm (such as the Titanium
FARO arm) an optical probe and/or a deformable material or device.
[0036]
A variety of tools
can be used to facilitate the implantation of the devices. The tools are guides
that assist in optimally positioning the device relative to the articular surface.
The design of tools and guides for use with the devices is derived from the
design of the device suitable for a particular joint. The tools can include
trial implants or surgical tools that partially or substantially conform to the
implantation site or joint cavity.
[0037]
Any of the repair
systems or prostheses described herein (e.g., the external surface) can
comprise a polymeric material or liquid metal. The polymeric material can be
attached to a metal or metal alloy. The polymeric material can be injected and,
for example, be self hardening or hardening when exposed to a chemical, energy
beam, light source, ultrasound and others. Further, any of the systems or
prostheses described herein can be adapted to receive injections, for example,
through an opening in the external surface of the cartilage replacement
material (e.g., an opening in the external surface terminates in a plurality of
openings on the bone surface). Bone cement, therapeutics, and/or other
bioactive substances can be injected through the opening(s). In certain
embodiments, it can be desirable to inject bone cement under pressure onto the
articular surface or subchondral bone or bone marrow in order to achieve
permeation of portions of the implantation site with bone cement. In addition,
any of the repair systems or prostheses described herein can be anchored in
bone marrow or in the subchondral bone itself. One or more anchoring extensions
(e.g., pegs, etc.) can extend through the bone and/or bone marrow.
[0038]
In some embodiments,
the cartilage replacement system can be implanted without breaching the
subchondral bone or with only few pegs or anchors extending into or through the
subchondral bone. This technique has the advantage of avoiding future implant
“settling” and osteolysis with resultant articular incongruity or implant
loosening or other complications.
[0039]
As will be
appreciated by those of skill in the art, suitable joints include knee,
shoulder, hip, vertebrae, intervertebral disks, elbow, ankle, wrist, fingers,
carpometacarpal, midfoot, and forefoot joints, to name a few. The techniques
described likewise are not limited to joints found in humans but can be
extended to joints in any mammal.
[0040]
These and other
embodiments of the subject invention will be apparent to those of skill in the
art in light of the disclosure herein.
BRIEF DESCRIPTION OF
THE DRAWINGS
[0041]
FIG.
1 A is a block diagram of a method for assessing a joint in need of
repair according to the invention wherein the existing joint surface is
unaltered, or substantially unaltered, prior to receiving the selected implant.
FIG. 1B is a block diagram of a method for assessing a joint in need of
repair according to the invention wherein the existing joint surface is
unaltered, or substantially unaltered, prior to designing an implant suitable
to achieve the repair.
[0042]
FIG. 2 is a
reproduction of a three-dimensional thickness map of the articular cartilage of
the distal femur. Three-dimensional thickness maps can be generated, for
example, from ultrasound, CT or MRI data. Dark holes within the substances of
the cartilage indicate areas of full thickness cartilage loss.
[0043]
FIG.
3 A illustrates an example of a Placido disk of concentrically
arranged circles of light. FIG. 3B illustrates an example of a projected
Placido disk on a surface of fixed curvature.
[0044]
FIG. 4 shows a
reflection resulting from a projection of concentric circles of light (Placido
Disk) on each femoral condyle, demonstrating the effect of variation in surface
contour on the reflected circles.
[0045]
FIG. 5 illustrates
an example of a 2D color-coded topographical map of an irregularly curved
surface.
[0046]
FIG. 6 illustrates
an example of a 3D color-coded topographical map of an irregularly curved
surface.
[0047]
FIG.
7 A-B are block diagrams of a method for assessing a joint in need of
repair according to the invention wherein the existing joint surface is altered
prior to receiving implant.
[0048]
FIG.
8 A is a perspective view of a joint implant of the invention
suitable for implantation at the tibial plateau of the knee joint. FIG.
8B is a top view of the implant of FIG. 8A. FIG. 8C is a
cross-sectional view of the implant of FIG. 8B along the lines C-C shown
in FIG. 8B. FIG. 8D is a cross-sectional view along the lines D-D shown in
FIG. 8B. FIG. 8E is a cross-sectional view along the lines E-E shown in
FIG. 8B. FIG. 8F is a side view of the implant of FIG. 8A. FIG. 8G is
a cross-sectional view of the implant of FIG. 8A shown implanted taken
along a plane parallel to the sagittal plane. FIG. 8H is a cross-sectional
view of the implant of FIG. 8A shown implanted taken along a plane
parallel to the coronal plane. FIG. 8I is a cross-sectional view of the
implant of FIG. 8A shown implanted taken along a plane parallel to the
axial plane. FIG. 8J shows a slightly larger implant that extends closer
to the bone medially (towards the edge of the tibial plateau) and anteriorly
and posteriorly. FIG. 8K is a side view of an alternate embodiment of the
joint implant of FIG. 8A showing an anchor. FIG. 8L is a bottom view
of an alternate embodiment of the joint implant of FIG. 8A showing an
anchor. FIG. 8M and N illustrate alternate embodiments of a two
piece implant from a front view and a side view.
[0049]
FIG.
9 A and B are perspective views of a joint implant suitable
for use on a condyle of the femur from the inferior and superior surface
viewpoints, respectively. FIG. 9C is a side view of the implant of FIG.
9A. FIG. 9D is a view of the inferior surface of the implant; FIG.
9E is a view of the superior surface of the implant and FIG. 9F is a
cross-section of the implant. FIG. 9G is a view of the superior surface of
a joint implant suitable for use on both condyles of the femur. FIG. 9H is
a perspective side view of the implant of FIG. 9G.
[0050]
FIG.
10 A is a side view of the acetabulum. FIG. 10B is a rotated
view of the proximal femur. FIG. 10C is a cross-sectional view of an
implant for a hip joint showing a substantially constant radius.
[0051]
FIG.
10 D is a cross-sectional view of an implant similar to that seen in
FIG. 10C with a round margin and an asymmetric radius.
[0052]
FIG. 11 A is
a cross-sectional view of an implant with a member extending into the fovea
capitis of the femoral head. Additional and alternative plan views are shown of
FIG. 11B showing the implant as a hemisphere, a partial hemisphere FIG.
11C and a rail FIG. 11D FIG. 11E is a view of an alternative
embodiment of an implant with a spoke arrangement.
[0053]
FIG.
12 A is a cross-sectional view of an implant with a member extending
into the acetabular fossa. FIG. 12B-E illustrate a variety of perspective
views wherein the implant is hemispherical, partially hemispherical, a rail and
a spoke.
[0054]
FIG.
13 A is a cross-sectional view of a dual component “mobile bearing”
implant showing a two piece construction and smooth mating surfaces. Plan views
are also shown showing dual components with two hemispheres, single hemisphere
with a rail or rail-like exterior component (i.e., hemispherical in one
dimension, but not in the remaining dimensions), single hemisphere with rail
interior structure, single hemisphere with spoke interior component, and single
hemisphere with spoke exterior component.
[0055]
FIG.
13 B-J are alternative embodiments of a dual component implant where
the interior surface of the exterior component has a nub that engages with in
indent on the exterior surface of the interior component. Additional variations
are also shown.
[0056]
FIG.
14 A is an alternative embodiment of an implant with a member
extending into the fovea capitis of the femoral head. FIG. 14B and FIG.
14C show cross-sectional embodiments, where one of the components forms a
hemisphere while the second component does not.
[0057]
FIG.
15 A is a cross-sectional view of a dual component “mobile bearing”
implant with a member extending into the acetabular fossa. FIG. 15B and
FIG. 15C show cross-sectional embodiments, where one of the components
forms a hemisphere while the second component does not.
[0058]
FIG.
16 A is a cross-sectional view of a triple component “mobile bearing”
implant. FIG. 16B-D are cross-sectional views of a triple component
“mobile bearing” implant that have one or more components forming a hemisphere
while at least one other component does not.
[0059]
FIG.
17 A is a cross-sectional view of a dual component “mobile bearing”
implant with a member extending into the acetabular fossa. FIG. 17B and
FIG. 17C show cross-sectional embodiments, where one of the components
forms a hemisphere while the second component does not.
[0060]
FIG.
18 A is a cross-sectional view of a dual component “mobile bearing”
implant with a member extending into the acetabular fossa. FIG. 18B is a
view from the top showing four fins on top of the member shown in FIG.
18A extending into the acetabular fossa on top of the acetabular
component.
[0061]
FIG.
19 A is a cross-sectional view of a dual component “mobile bearing”
implant with a member extending into the fovea capitis of the femoral head.
FIG. 19B is a cross-sectional view of a dual component fixed implant.
[0062]
FIG.
20 A is a cross-sectional view of an implant with varying radii and
thickness for a hip joint. FIG. 20B is a cross-sectional view of an
implant with varying radii and thickness for a hip joint. FIG. 20C is a
cross-sectional view of an implant with varying radii and thickness for a hip
joint. FIG. 20D is a cross-sectional view of an implant for a hip joint
with a lip extending inferiorly and superiorly.
[0063]
FIG.
21 A is a frontal view of the osseous structures in the shoulder
joint such as the clavicle, scapula, glenoid fossa, acromion, coracoid process
and humerus. FIG. 21B is a view of an arthroplasty device placed between
the humeral head and the glenoid fossa. FIG. 21C is an oblique frontal
cross-sectional view of an arthroplasty device with the humeral surface
conforming substantially to the shape of the humeral head and the glenoid
surface conforming substantially to the shape of the glenoid. FIG. 21D is
an axial cross-sectional view of an arthroplasty device with the humeral
surface conforming substantially to the shape of the humeral head and the
glenoid surface conforming substantially to the shape of the glenoid. FIG.
21E is an oblique frontal view of the shoulder demonstrating the articular
cartilage and the superior and inferior glenoid labrum. FIG. 21F is an
axial view of the shoulder demonstrating the articular cartilage and the
anterior and posterior glenoid labrum. FIG. 21G is an oblique frontal
cross-sectional view of an arthroplasty device with the humeral surface
conforming substantially to the shape of the humeral head and the glenoid
surface conforming substantially to the shape of the glenoid and the glenoid
labrum. FIG. 21H is an axial cross-sectional view of an arthroplasty with
the humeral surface conforming substantially to the shape of the humeral head
and the glenoid surface conforming substantially to the shape of the glenoid
and the glenoid labrum. FIG. 21I is an oblique frontal cross-sectional
view of an arthroplasty device with the humeral surface conforming
substantially to the shape of the humeral head and the glenoid surface
conforming substantially to the shape of the glenoid. A lip is shown extending
superiorly and/or inferiorly which provides stabilization over the glenoid.
FIG. 21J is an axial cross-sectional view of an arthroplasty device with
the humeral surface conforming substantially to the shape of the humeral head
and the glenoid surface conforming substantially to the shape of the glenoid. A
lip is shown extending anteriorly and/or posteriorly which provides
stabilization over the glenoid. FIG. 21K is an oblique frontal
cross-sectional view of a dual component, “mobile-bearing” arthroplasty device
with the humeral surface conforming substantially to the shape of the humeral
head and the glenoid surface conforming substantially to the shape of the
glenoid.
[0064]
FIG.
21 L is an axial cross-sectional view of a dual component,
“mobile-bearing” arthroplasty device with a humeral conforming surface that
conforms to the shape of the humeral head and a glenoid conforming surface that
conforms to the shape of the glenoid. FIG. 21M is an alternate view of a
dual component, “mobile-bearing” arthroplasty device with a humeral conforming
surface that conforms to the shape of the humeral head and a glenoid conforming
surface that conforms to the shape of the glenoid. The device has a nub on the
surface of the first component that mates with an indent on the surface of the
second component to enhance joint movement.
[0065]
FIG.
21 N is an oblique frontal cross-sectional view of a dual component,
“mobile-bearing” arthroplasty device. FIG. 21O is an oblique frontal cross-sectional
view of a dual component, “mobile-bearing” arthroplasty device. FIG.
21P and Q are cross-sectional views of alternate embodiments of
the dual mobile bearing device shown in FIG. 21O.
[0066]
FIG. 22 is an
oblique longitudinal view through the elbow joint demonstrating the distal
humerus, the olecranon and the radial head. The cartilaginous surfaces are also
shown.
[0067]
FIG.
23 A is a longitudinal view through the wrist joint demonstrating the
distal radius, the ulna and several of the carpal bones with an arthroplasty
system in place. FIG. 23B is a longitudinal view through the wrist joint
demonstrating the distal radius, the ulna and several of the carpal bones with
an arthroplasty system in place. FIG. 23C is a longitudinal view through
the wrist joint demonstrating the distal radius, the ulna and several of the
carpal bones with an arthroplasty system in place. FIG. 23D is a
longitudinal view of a dual component, “mobile-bearing” arthroplasty device
suitable for the wrist. FIG. 23E is a longitudinal view of another dual
component arthroplasty device, in this case without lips. FIG. 23F is a
longitudinal view of a dual component, “mobile-bearing” arthroplasty device.
[0068]
FIG. 24 is a sagittal
view through a finger. An arthroplasty device is shown interposed between the
metacarpal head and the base of the proximal phalanx.
[0069]
FIG.
25 A is a sagittal view through the ankle joint demonstrating the
distal tibia, the talus and calcaneus and the other bones with an arthroplasty
system in place. FIG. 25B is a coronal view through the ankle joint
demonstrating the distal tibia, the distal fibula and the talus. An
arthroplasty device is shown interposed between the distal tibia and the talar
dome. FIG. 25C is a sagittal view through the ankle joint demonstrating
the distal tibia, the talus and calcaneus and the other bones. The
cartilaginous surfaces are also shown. An arthroplasty device is shown
interposed between the distal tibia and the talar dome. FIG. 25D is a
coronal view through the ankle joint demonstrating the distal tibia, the distal
fibula and the talus. An arthroplasty device is shown interposed between the
distal tibia and the talar dome.
[0070]
FIG. 26 is a
sagittal view through a toe. An arthroplasty device is shown interposed between
the metatarsal head and the base of the proximal phalanx.
[0071]
FIG.
27 A-D are block diagrams of method steps employed while implanting
an device of the invention into a target joint.
[0072]
FIG. 28 is a plan
view of an implant guide tool suitable for use implanting the device shown in
FIG. 8 L
[0073]
FIG.
29 A and B are a plan views of an implant guide tool
suitable for use implanting the device shown in FIG. 9B.
DETAILED DESCRIPTION
OF THE INVENTION
[0074]
The following
description is presented to enable any person skilled in the art to make and
use the invention. Various modifications to the embodiments described will be
readily apparent to those skilled in the art, and the generic principles
defined herein can be applied to other embodiments and applications without
departing from the spirit and scope of the present invention as defined by the
appended claims. Thus, the present invention is not intended to be limited to
the embodiments shown, but is to be accorded the widest scope consistent with
the principles and features disclosed herein. To the extent necessary to
achieve a complete understanding of the invention disclosed, the specification
and drawings of all issued patents, patent publications, and patent
applications cited in this application are incorporated herein by reference.
[0075]
As will be
appreciated by those of skill in the art, the practice of the present invention
employs, unless otherwise indicated, conventional methods of x-ray imaging and
processing, x-ray tomosynthesis, ultrasound including A-scan, B-scan and
C-scan, computed tomography (CT scan), magnetic resonance imaging (MRI),
optical coherence tomography, single photon emission tomography (SPECT) and
positron emission tomography (PET) within the skill of the art. Such techniques
are explained fully in the literature and need not be described herein. See,
e.g., X-Ray Structure Determination: A Practical Guide, 2nd Edition, editors
Stout and Jensen, 1989, John Wiley & Sons, publisher; Body CT: A Practical
Approach, editor Slone, 1999, McGraw-Hill publisher; X-ray Diagnosis: A
Physician's Approach, editor Lam, 1998 Springer-Verlag, publisher; and Dental
Radiology: Understanding the X-Ray Image, editor Laetitia Brocklebank 1997, Oxford
University Press publisher.
I. Dual or Multiple
Surface Assessment of the Joint
[0076]
The invention
allows, among other things, a practitioner to evaluate and treat defects to
joints resulting from, for example, joint disease, cartilage degeneration,
osteoarthritis, seropositive and seronegative arthritides, bone damages,
cartilage damage, trauma, and/or degeneration due to overuse or age. The size,
volume and shape of the area of interest can include only the region of
cartilage that has the defect, but preferably can also include contiguous parts
of the cartilage surrounding the cartilage defect. Moreover, the size, volume
and shape of the area of interest can include subchondral bone, bone marrow and
other articular structures, e.g. menisci, ligaments and tendons.
[0077]
FIG.
1 A is a flow chart showing steps taken by a practitioner in
assessing a joint. First, a practitioner obtains a measurement of a target
joint 10. The step of obtaining a measurement can be accomplished by
taking an image of the joint. This step can be repeated, as necessary, 11 to
obtain a plurality of images in order to further refine the joint assessment
process. Once the practitioner has obtained the necessary measurements, the
information is used to generate a model representation of the target joint
being assessed 30. This model representation can be in the form of a
topographical map or image. The model representation of the joint can be in
one, two, or three dimensions. It can include a physical model. More than one
model can be created 31, if desired. Either the original model, or a
subsequently created model, or both can be used. After the model representation
of the joint is generated 30, the practitioner can optionally generate a
projected model representation of the target joint in a
corrected condition 40. Again, this step can be repeated 41, as
necessary or desired. Using the difference between the topographical condition
of the joint and the projected image of the joint, the practitioner can then
select a joint implant 50 that is suitable to achieve the
corrected joint anatomy. As will be appreciated by those of skill in the art,
the selection process 50 can be repeated 51 as often
as desired to achieve the desired result.
[0078]
As will be
appreciated by those of skill in the art, the practitioner can proceed directly
from the step of generating a model representation of the target joint 30 to
the step of selecting a suitable joint replacement implant 50 as
shown by the arrow 32. Additionally, following selection of suitable joint
replacement implant 50, the steps of obtaining measurement of target
joint 10, generating model representation of target joint 30 and
generating projected model 40, can be repeated in series or parallel
as shown by the flow 24, 25, 26.
[0079]
FIG.
1 B is an alternate flow chart showing steps taken by a practitioner
in assessing a joint. First, a practitioner obtains a measurement of a target
joint 10. The step of obtaining a measurement can be accomplished by
taking an image of the joint. This step can be repeated, as necessary, 11 to
obtain a plurality of images in order to further refine the joint assessment
process. Once the practitioner has obtained the necessary measurements, the
information is used to generate a model representation of the target joint
being assessed 30. This model representation can be in the form of a
topographical map or image. The model representation of the joint can be in
one, two, or three dimensions. The process can be repeated 31 as
necessary or desired. It can include a physical model. After the model
representation of the joint is assessed 30, the practitioner can
optionally generate a projected model representation of the target joint of the
joint in a corrected condition 40. This step can be repeated 41 as
necessary or desired. Using the difference between the topographical condition
of the joint and the projected image of the joint, the practitioner can then
design a joint implant 52 that is suitable to achieve the
corrected joint anatomy, repeating the design process 53 as
often as necessary to achieve the desired implant design. The practitioner can
also assess whether providing additional features, such as lips, pegs, or
anchors, will enhance the implants' performance in the target joint.
[0080]
As will be
appreciated by those of skill in the art, the practitioner can proceed directly
from the step of generating a model representation of the target joint 30 to
the step of designing a suitable joint replacement implant 52 as
shown by the arrow 38. Similar to the flow shown above, following the
design of a suitable joint replacement implant 52, the steps of
obtaining measurement of target joint 10, generating model representation
of target joint 30 and generating projected model 40, can
be repeated in series or parallel as shown by the flow 42, 43, 44.
[0081]
The joint implant
selected or designed achieves anatomic or near anatomic fit with the existing
surface of the joint while presenting a mating surface for the opposing joint
surface that replicates the natural joint anatomy. In this instance, both the
existing surface of the joint can be assessed as well as the desired resulting
surface of the joint. This technique is particularly useful for implants that
are not anchored into the bone.
[0082]
FIG. 2 illustrates a
color reproduction of a 3-dimensional thickness map of the articular cartilage
of the distal femur. Thee-dimensional thickness maps can be generated, for
example, from ultrasound, CT, or MRI data. Dark holes within the substance of
the cartilage indicate areas of full thickness cartilage loss. From the
3-dimensional thickness map a determination can be made of the size and shape
of cartilage damage.
[0083]
As will be
appreciated by those of skill in the art, size, curvature and/or thickness
measurements can be obtained using any suitable technique. For example, one
dimensional, two dimensional, and/or in three dimensional measurements can be
obtained using suitable mechanical means, laser devices, electromagnetic or
optical tracking systems, molds, materials applied to the articular surface
that harden and “memorize the surface contour,” and/or one or more imaging
techniques known in the art. Measurements can be obtained non-invasively and/or
intraoperatively (e.g., using a probe or other surgical device). As will be
appreciated by those of skill in the art, the thickness of the repair device
can vary at any given point depending upon the depth of the damage to the
cartilage and/or bone to be corrected at any particular location on an articular
surface.
[0084]
A. Imaging
Techniques
[0085]
As will be
appreciated by those of skill in the art, imaging techniques suitable for
measuring thickness and/or curvature (e.g., of cartilage and/or bone) or size
of areas of diseased cartilage or cartilage loss include the use of x-rays,
magnetic resonance imaging (MRI), computed tomography scanning (CT, also known
as computerized axial tomography or CAT), optical coherence tomography, SPECT,
PET, ultrasound imaging techniques, and optical imaging techniques. (See, also,
International patent Publication WO 02/22014 to Alexander, et al., published
Mar. 21, 2002; U.S. Pat. No. 6,373,250 to Tsoref et al., issued Apr. 16, 2002;
and Vandeberg et al. (2002) Radiology 222:430-436). Contrast or other
enhancing agents can be used using any route of administration, e.g.
intravenous, intra-articular, etc.
[0086]
In certain
embodiments, CT or MRI is used to assess tissue, bone, cartilage and any
defects therein, for example cartilage lesions or areas of diseased cartilage,
to obtain information on subchondral bone or cartilage degeneration and to
provide morphologic or biochemical or biomechanical information about the area
of damage. Specifically, changes such as fissuring, partial or full thickness
cartilage loss, and signal changes within residual cartilage can be detected
using one or more of these methods. For discussions of the basic NMR principles
and techniques, see MRI Basic Principles and Applications, Second Edition, Mark
A. Brown and Richard C. Semelka, Wiley-Liss, Inc. (1999). For a discussion of
MRI including conventional T1 and T2-weighted spin-echo imaging, gradient
recalled echo (GRE) imaging, magnetization transfer contrast (MTC) imaging,
fast spin-echo (FSE) imaging, contrast enhanced imaging, rapid acquisition
relaxation enhancement, (RARE) imaging, gradient echo acquisition in the steady
state, (GRASS), and driven equilibrium Fourier transform (DEFT) imaging, to
obtain information on cartilage, see Alexander, et al., WO 02/22014. Thus, in
preferred embodiments, the measurements obtained are based on three-dimensional
images obtained of the joint as described in Alexander, et al., WO 02/22014 or
sets of two-dimensional images ultimately yielding 3D information.
Two-dimensional, three-dimensional images, or maps, of the cartilage alone or
in combination with a movement pattern of the joint, e.g. flexion-extension,
translation and/or rotation, can be obtained. Three-dimensional images can
include information on movement patterns, contact points, contact zone of two
or more opposing articular surfaces, and movement of the contact point or zone
during joint motion. Two and three-dimensional images can include information
on biochemical composition of the articular cartilage. In addition, imaging
techniques can be compared over time, for example to provide up-to-date
information on the shape and type of repair material needed.
[0087]
Any of the imaging
devices described herein can also be used intra-operatively (see, also below),
for example using a hand-held ultrasound and/or optical probe to image the
articular surface intra-operatively.
[0088]
B. Intraoperative
Measurements
[0089]
Alternatively, or in
addition to, non-invasive imaging techniques described above, measurements of
the size of an area of diseased cartilage or an area of cartilage loss,
measurements of cartilage thickness and/or curvature of cartilage or bone can
be obtained intraoperatively during arthroscopy or open arthrotomy.
Intraoperative measurements may or may not involve actual contact with one or
more areas of the articular surfaces.
[0090]
Devices suitable for
obtaining intraoperative measurements of cartilage or bone or other articular
structures, and to generate a topographical map of the surface include but are
not limited to, Placido disks and laser interferometers, and/or deformable
materials or devices. (See, for example, U.S. Pat. No. 6,382,028 to Wooh et
al., issued May 17, 2002; U.S. Pat. No. 6,057,927 to Levesque et al., issued
May 2, 2000; U.S. Pat. No. 5,523,843 to Yamane et al. issued Jun. 4, 1996; U.S.
Pat. No. 5,847,804 to Sarver et al. issued Dec. 8, 1998; and U.S. Pat. No.
5,684,562 to Fujeda, issued Nov. 4, 1997).
[0091]
FIG.
3 A illustrates a Placido disk of concentrically arranged circles of
light. The concentric arrays of the Placido disk project well-defined circles
of light of varying radii, generated either with laser or white light
transported via optical fiber. The Placido disk can be attached to the end of
an endoscopic device (or to any probe, for example a hand-held probe) so that
the circles of light are projected onto the cartilage surface. FIG.
3B illustrates an example of a Placido disk projected onto the surface of
a fixed curvature. One or more imaging cameras can be used (e.g., attached to
the device) to capture the reflection of the circles. Mathematical analysis is
used to determine the surface curvature. The curvature can then, for example,
be visualized on a monitor as a color-coded, topographical map of the cartilage
surface. Additionally, a mathematical model of the topographical map can be
used to determine the ideal surface topography to replace any cartilage defects
in the area analyzed. This computed, ideal surface can then also be visualized
on the monitor such as the 3-dimensional thickness map shown in FIG. 2, and can
be used to select the curvature of the surfaces of the replacement material or
regenerating material.
[0092]
FIG. 4 shows a
reflection resulting from the projection of concentric circles of light
(Placido disk) on each femoral condyle, demonstrating the effect of variation
in surface contour on reflected circles.
[0093]
Similarly a laser
interferometer can also be attached to the end of an endoscopic device. In
addition, a small sensor can be attached to the device in order to determine
the cartilage surface or bone curvature using phase shift interferometry,
producing a fringe pattern analysis phase map (wave front) visualization of the
cartilage surface. The curvature can then be visualized on a monitor as a color
coded, topographical map of the cartilage surface. Additionally, a mathematical
model of the topographical map can be used to determine the ideal surface
topography to replace any cartilage or bone defects in the area analyzed. This
computed, ideal surface, or surfaces, can then be visualized on the monitor,
and can be used to select the curvature, or curvatures, of the replacement
cartilage.
[0094]
One skilled in the
art will readily recognize that other techniques for optical measurements of
the cartilage surface curvature can be employed without departing from the
scope of the invention. For example, a 2-dimentional or 3-dimensional map, such
as that shown in FIG. 5 and FIG. 6 can be generated.
[0095]
Mechanical devices
(e.g., probes) can also be used for intraoperative measurements, for example,
deformable materials such as gels, molds, any hardening materials (e.g.,
materials that remain deformable until they are heated, cooled, or otherwise
manipulated). See, e.g., WO 02/34310 to Dickson et al., published May 2, 2002.
For example, a deformable gel can be applied to a femoral condyle. The side of
the gel pointing towards the condyle can yield a negative impression of the
surface contour of the condyle. The negative impression can then be used to
determine the size of a defect, the depth of a defect and the curvature of the
articular surface in and adjacent to a defect. This information can be used to
select a therapy, e.g. an articular surface repair system. In another example,
a hardening material can be applied to an articular surface, e.g. a femoral
condyle or a tibial plateau. The hardening material can remain on the articular
surface until hardening has occurred. The hardening material can then be
removed from the articular surface. The side of the hardening material pointing
towards the articular surface can yield a negative impression of the articular
surface. The negative impression can then be used to determine the size of a
defect, the depth of a defect and the curvature of the articular surface in and
adjacent to the defect. This information can then be used to select a therapy,
e.g. an articular surface repair system. In some embodiments, the hardening
system can remain in place and form the actual articular surface repair system.
[0096]
In certain
embodiments, the deformable material comprises a plurality of individually
moveable mechanical elements. When pressed against the surface of interest,
each element can be pushed in the opposing direction and the extent to which it
is pushed (deformed) can correspond to the curvature of the surface of
interest. The device can include a brake mechanism so that the elements are
maintained in the position that conforms to the surface of the cartilage and/or
bone. The device can then be removed from the patient and analyzed for
curvature. Alternatively, each individual moveable element can include markers
indicating the amount and/or degree it is deformed at a given spot. A camera
can be used to intra-operatively image the device and the image can be saved
and analyzed for curvature information. Suitable markers include, but are not
limited to, actual linear measurements (metric or imperial), different colors
corresponding to different amounts of deformation and/or different shades or
hues of the same color(s). Displacement of the moveable elements can also be
measured using electronic means.
[0097]
Other devices to measure
cartilage and subchondral bone intraoperatively include, for example,
ultrasound probes. An ultrasound probe, preferably handheld, can be applied to
the cartilage and the curvature of the cartilage and/or the subchondral bone
can be measured. Moreover, the size of a cartilage defect can be assessed and
the thickness of the articular cartilage can be determined. Such ultrasound
measurements can be obtained in A-mode, B-mode, or C-mode. If A-mode
measurements are obtained, an operator can typically repeat the measurements
with several different probe orientations, e.g. mediolateral and
anteroposterior, in order to derive a three-dimensional assessment of size,
curvature and thickness.
[0098]
One skilled in the
art will easily recognize that different probe designs are possible using the
optical, laser interferometry, mechanical and ultrasound probes. The probes are
preferably handheld. In certain embodiments, the probes or at least a portion
of the probe, typically the portion that is in contact with the tissue, can be
sterile. Sterility can be achieved with use of sterile covers, for example
similar to those disclosed in WO 99/08598A1 to Lang, published Feb. 25, 1999.
[0099]
Analysis on the
curvature of the articular cartilage or subchondral bone using imaging tests
and/or intraoperative measurements can be used to determine the size of an area
of diseased cartilage or cartilage loss. For example, the curvature can change
abruptly in areas of cartilage loss. Such abrupt or sudden changes in curvature
can be used to detect the boundaries of diseased cartilage or cartilage
defects.
[0100]
II. Single Surface
Assessment of a Joint
[0101]
Turning now to FIG.
7 A, a block diagram is provided showing steps for performing a single
surface assessment of the joint. As with FIG. 1A and B an image
or measurement is obtained of the target joint 60. Thereafter a
measurement is taken to assist in selecting an appropriate device to correct
the defect 70. The measuring or imaging steps can be repeated as
desired to facilitate identifying the most appropriate device 80 to
repair the defect. Once the measurement or measurements have been taken, a
device is selected for correcting the defect 90. In this instance,
only one surface of the joint is replicated. This technique is particularly
useful for implants that include mechanisms for anchoring the implant into the
bone. Thus, the implant has at least one surface that replicates a joint
surface with at least a second surface that communicates with some or all of
the articular surface or bone of the damaged joint to be repaired.
[0102]
As will be
appreciated by those of skill in the art, the practitioner can proceed directly
from the step of measuring the joint defect 70 to the step of
selecting a suitable device to repair the defect 90 as shown by
the arrow 38. Further any, or all, of the steps of obtaining a
measurement of a target joint 60, measuring a joint defect 70,
identifying device suitable to repair the defect 80, selecting a
device to repair the defect 90, can be repeated one or more
times 61, 71, 81, 91, as desired.
[0103]
Similar to the flow
shown above, following the selection of a device to repair
the defect 90, the steps of obtaining a measurement of a target
joint 60, measuring a joint defect 70, identifying device suitable
to repair the defect 80, can be repeated in series or parallel as
shown by the flow 65, 66, 67.
[0104]
FIG.
7 B shows an alternate method. A block diagram is provided showing
steps for performing a single surface assessment of the joint. As with FIG.
1A and B an image or measurement is obtained of the target
joint 60. Thereafter a measurement is taken to assist in selecting an
appropriate device to correct the defect 70. The measuring or imaging
steps can be repeated 71 as desired to facilitate identifying the
most appropriate device 80 to repair the defect. Once the
measurement or measurements have been taken, a device is manufactured for
correcting the defect 92.
[0105]
As will be
appreciated by those of skill in the art, the practitioner can proceed directly
from the step of measuring the joint defect 70 to the step of
manufacturing a device to repair the defect 92 as shown by
the arrow 39. Further any, or all, of the steps of obtaining a
measurement of a target joint 60, measuring a joint defect 70,
identifying device suitable to repair the defect 80, manufacturing a
device to repair the defect 92, can be repeated one or more
times 61, 71, 81, 93, as desired.
[0106]
Similar to the flow
shown above, following the manufacture of a device to repair
the defect 92, the steps of obtaining a measurement of a target
joint 60, measuring a joint defect 70, identifying device
suitable to repair the defect 80, can be repeated in series or
parallel as shown by the flow 76, 77, 78.
[0107]
Various methods are
available to facilitate the modeling the joint during the single surface
assessment. For example, using information on thickness and curvature of the
cartilage, a model of the surfaces of the articular cartilage and/or of the
underlying bone can be created for any joint. The model representation of the
joint can be in one, two, or three dimensions. It can include a physical model.
This physical model can be representative of a limited area within the joint or
it can encompass the entire joint.
[0108]
More specifically,
in the knee joint, the physical model can encompass only the medial or lateral
femoral condyle, both femoral condyles and the notch region, the medial tibial
plateau, the lateral tibial plateau, the entire tibial plateau, the medial
patella, the lateral patella, the entire patella or the entire joint. The
location of a diseased area of cartilage can be determined, for example using a
3D coordinate system or a 3D Euclidian distance transform as described in WO
02/22014 to Alexander, et al. or a LaPlace transform.
[0109]
In this way, the
size of the defect to be repaired can be accurately determined. As will be
apparent, some, but not all, defects can include less than the entire
cartilage. The thickness of the normal or only mildly diseased cartilage
surrounding one or more cartilage defects is measured. This thickness
measurement can be obtained at a single point or a plurality of points. The
more measurements that are taken, the more refined and accurate the measurement
becomes. Thus, measurements can be taken at, for example, 2 points, 4-6 points,
7-10 points, more than 10 points or over the length of the entire remaining
cartilage. Two-dimensional and three-dimensional measurements can be obtained.
Furthermore, once the size of the defect is determined, an appropriate therapy
(e.g., implant or an implant replacing an area equal to or slightly greater
than the diseased cartilage covering one or more articular surfaces) can be
selected such that as much as possible of the healthy, surrounding tissue is
preserved.
[0110]
Alternatively, the
curvature of the articular surface or the underlying bone can be measured to
design and/or shape the repair material. In this instance, both the thickness
of the remaining cartilage and the curvature of the articular surface can be
measured to design and/or shape the repair material. Alternatively, the
curvature of the subchondral bone can be measured and the resultant
measurement(s) can be used to design, produce, select and/or shape a cartilage
replacement material.
[0111]
III. Joint Devices
[0112]
The present device
is a prosthesis. The form of the prosthesis or device is determined by
projecting the contour of the existing cartilage and/or bone to effectively
mimic aspects of the natural articular structure. The device substantially
restores the normal joint alignment and/or provides a congruent or
substantially congruent surface to the original or natural articular surface of
an opposing joint surface that it mates with. Further, it can essentially
eliminate further degeneration because the conforming surfaces of the device
provide an anatomic or near anatomic fit with the existing articular surfaces
of the joint. Insertion of the device is done via a small (e.g., 3 cm to 5 cm)
incision and no bone resection or mechanical fixation of the device is
required. However, as will be appreciated by those of skill in the art,
additional structures can be provided, such as a cross-bar, fins, pegs, teeth
(e.g., pyramidal, triangular, spheroid, or conical protrusions), or pins, that
enhance the devices' ability to seat more effectively on the joint surface.
Osteophytes or other structures that interfere with the device placement are
easily removed. By occupying the joint space in an anatomic or near anatomic
fit, the device improves joint stability and restores normal or near normal
mechanical alignment of the joint.
[0113]
The precise
dimensions of the devices described herein can be determined by obtaining and
analyzing images of a particular subject and designing a device that
substantially conforms to the subject's joint anatomy (cartilage and/or bone)
while taking into account the existing articular surface anatomy as described
above. Thus, the actual shape of the present device can be tailored to the individual.
[0114]
A prosthetic device
of the subject invention can be a device suitable for minimally invasive,
surgical implantation without requiring bone resection. The device can, but
need not be, affixed to the bone. For example, in the knee the device can be
unicompartmental, i.e., positioned within a compartment in which a portion of
the natural meniscus is ordinarily located. The natural meniscus can be
maintained in position or can be wholly or partially removed, depending upon
its condition. Under ordinary circumstances, pieces of the natural meniscus
that have been torn away are removed, and damaged areas can be trimmed, as
necessary. Alternatively, all of the remaining meniscus can be removed. This
can be done via the incision used for insertion of the device. For many of the
implants, this can also be done arthroscopically making an incision that is
1-15 cm in length, but more preferably 1-8 cm in length, and even more
preferably 1-4 cm.
[0115]
The implants
described herein can have varying curvatures and radii within the same plane,
e.g. anteroposterior or mediolateral or superoinferior or oblique planes, or
within multiple planes. In this manner, the articular surface repair system can
be shaped to achieve an anatomic or near anatomic alignment between the implant
and the implant site. This design not only allows for different degrees of
convexity or concavity, but also for concave portions within a predominantly
convex shape or vice versa. The surface of the implant that mates with the
joint being repaired can have a variable geography that can be a function of
the physical damage to the joint surface being repaired. Although, persons of
skill in the art will recognize that implants can be crafted based on typical
damage patterns. Implants can also be crafted based on the expected normal
congruity of the articular structures before the damage has occurred.
[0116]
Moreover, implants
can be crafted accounting for changes in shape of the opposing surfaces during
joint motion. Thus, the implant can account for changes in shape of one or more
articular surface during flexion, extension, abduction, adduction, rotation,
translation, gliding and combinations thereof.
[0117]
The devices
described herein are preferably marginally translatable and self-centering.
Thus, during natural articulation of a joint, the device is allowed to move
slightly, or change its position as appropriate to accommodate the natural
movement of the joint. The device does not, however, float freely in the joint.
Further, upon translation from a first position to a second position during
movement of a joint, the device tends to returns to substantially its original
position as the movement of the joint is reversed and the prior position is
reached. As a result, the device tends not to progressively “creep” toward one
side of the compartment in which it is located. The variable geography of the
surface along with the somewhat asymmetrical shape of the implant facilitates
the self-centering behavior of the implant.
[0118]
The device can also
remain stationary over one of the articular surface. For example, in a knee
joint, the device can remain centered over the tibia while the femoral condyle
is moving freely on the device. The somewhat asymmetrical shape of the implant
closely matched to the underlying articular surface helps to achieve this kind
of stabilization over one articular surface.
[0119]
The motion within
the joint of the devices described herein can optionally, if desired, be
limited by attachment mechanisms. These mechanisms can, for example, allow the
device to rotate, but not to translate. It can also allow the device to
translate in one direction, while preventing the device from translating into
another direction. The mechanisms can furthermore fix the devices within the joint
while allowing the device to tilt. Suitable attachment mechanisms include
ridges, pegs, pins, cross-members, teeth and protrusions. The configuration of
these mechanisms can be parallel to one another, or non-parallel in
orientation. The mechanisms can be pyramidal, triangular, spheroid, conical, or
any shape that achieves the result. One or more attachment mechanism can be
provided. Where more than one mechanism is provided, the mechanisms can cover
the entire surface of the device, or a portion of the surface. Additional
stabilization mechanisms can be provided such as ridges, lips and thickenings
along all or a portion of a peripheral surface.
[0120]
The implant shape
can also incorporate the shape of the joint on which it is position, such as
portions of the tibial spines. Adding conformity with the tibial spines, e.g.
the base of the tibial spines, can help in stabilizing the implant relative to
the tibial plateau.
[0121]
The implant height
or profile selected can be chosen to alter the load bearing ability relative to
the joint. Additionally the implant height can be adjusted to account for
anatomic malalignment of bones or articular structures. Additionally, for any
of the implants taught herein in the presence of ligamentous laxity, the
implant height, profile or other dimension can be adjusted to allow tightening
of the ligament apparatus to improve the function. This occurs preferably
without substantially interfering with axis alignment of the bones. Typically,
the joints of are able to withstand up to 100% of the shear force exerted on
the joint in motion.
[0122]
The implants of the
invention typically restore joint mobility up to 99.9% of natural mobility of
the joint for a particular subject. For example, in the case of the knee
overall articulation typically ranges from 0 to 140°. Currently available
solutions typically restore articulation in a range substantially less than
99.9%, while implants of the present invention typically restore the range of
motion to between 95-99.9% of normal range of motion for the patient.
[0123]
Ranges of motion for
joints of the hands and arms for a healthy male obtained from National
Institute of Standards and Technology (http://ovrt.nist.gov) are described in
T ABLE 1.
TABLE 1
RANGE OF MOVEMENT OF HAND AND ARM JOINTS
Joint
Movement |
Range
(degree) Average |
Range
(degree) S.D. |
|
Wrist
Flexion |
90 |
12 |
|
Wrist
Extension |
99 |
13 |
|
Wrist
Adduction |
27 |
9 |
|
Wrist
Abduction |
47 |
7 |
|
Forearm
Supination |
113 |
22 |
|
Forearm
Pronation |
77 |
24 |
|
Elbow
Flexion |
142 |
10 |
|
Shoulder
Flexion |
188 |
12 |
Shoulder
Extension |
61 |
14 |
|
Shoulder
Adduction |
48 |
9 |
|
Shoulder
Abduction |
134 |
17 |
[0124]
Ranges of motion for
joints of the foot and leg for a healthy male obtained from National Institute
of Standards and Technology (http://ovrt.nist.gov) are described in
T ABLE 2.
TABLE 2
RANGE OF MOVEMENT OF FOOT AND LEG JOINTS
|
Joint
Movement |
Range
(degree) Average |
Range
(degree) S.D. |
|
Ankle
Flexion |
35 |
7 |
Ankle
Extension |
38 |
12 |
Ankle
Adduction |
24 |
9 |
|
Ankle
Abduction |
23 |
7 |
|
Knee
Flexion - Standing |
113 |
13 |
|
Knee
Flexion - Kneeling |
159 |
9 |
|
Knee
Flexion - Prone |
125 |
10 |
|
Knee
Rotation - Medial |
35 |
12 |
|
Knee
Rotation - |
43 |
12 |
|
Hip
Flexion |
113 |
13 |
Hip
Adduction |
31 |
12 |
Hip
Abduction |
53 |
12 |
|
Hip
Rotation – Sitting (medial) |
31 |
9 |
|
Hip
Rotation – Sitting (lateral) |
30 |
9 |
|
Hip
Rotation – Prone (medial) |
39 |
10 |
|
Hip
Rotation – Prone (lateral) |
34 |
10 |
[0125]
Implants of the
present invention should typically restore the range of motion for one or more
of the measurements in Tables 1 and 2 for any joint to between 60-99.9% of
normal range of motion for the patient and more preferably between 95-99.9% of
normal range of motion for the patient.
[0126]
As discussed in more
detail below, any of the devices taught herein can be manufactured in a variety
of ways such that the device is, for example, expands after insertion.
Expansion can either be automatic, semi-automatic or upon adjustment by the
user.
[0127]
Turning now to
illustrative examples of joint implants according to the scope and teachings of
the invention.
[0128]
A. The Knee
[0129]
FIG.
8 A shows a perspective view of a joint implant 100 of
the invention suitable for implantation at the tibial plateau of the knee
joint. As shown in FIG. 8A, the implant is generated using a dual surface
assessment, as described above with respect to FIG. 1A and B.
[0130]
The implant 100 has
an upper surface 102 and a lower surface 104 and
a peripheral edge 106. The upper surface 102 is formed
so that it forms a mating surface for receiving the opposing joint surface; in
this instance partially concave to receive the femur. The concave surface can
be variably concave such that it presents a surface to the opposing joint
surface that approximates the mating surface of the joint it corrects.
The lower surface 104 has a convex surface matches, or nearly
matches, the tibial plateau of the joint such that it creates an anatomic or near
anatomic fit with the tibial plateau. Depending on the shape of the tibial
plateau, the lower surface can be partially convex. Thus, the lower
surface 104 presents a surface to the tibial plateau that fits within
the existing surface. As will be appreciated by those of skill in the art, the
convex surface of the lower surface 104 need not be perfectly
convex. Rather, the lower surface 104 is more likely consist of
convex and concave elements to fit within the existing surface of the tibial
plateau. Thus the surface is essentially variably convex and concave.
[0131]
FIG.
8 B shows a top view of the joint implant of FIG. 8A. As shown in
FIG. 8B the exterior shape 108 of the implant can be
elongated. The elongated form can take a variety of shapes including
elliptical, quasi-elliptical, race-track, etc. However, as will be appreciated
the exterior dimension is typically irregular thus not forming a true geometric
ellipse. As will be appreciated by those of skill in the art, the actual
exterior shape of an implant can vary depending on the nature of the joint
defect to be corrected. Thus the ratio of the length L to the width W can vary
from, for example, between 0.5 to 1.5, and more specifically from 0.25 to 2.0.
As further shown in FIG. 8B, the length across an axis of
the implant 100 varies when taken at points along the width of
the implant. For example, as shown in FIG. 8B, L1≠L2 ≠L3.
[0132]
Turning now to FIG.
8 C-E, a cross-section of the implant shown in FIG. 8B is depicted
along the lines of C-C, D-D, and E-E is shown. The implant has a thickness t1,
t2 and t3 respectively. As illustrated by the cross-sections, the thickness of
the implant varies along its length L. The actual thickness at a particular
location of the implant 100 is a function of the thickness of
the cartilage and/or bone to be replaced and the joint mating surface to be
replicated. Further, the profile of the implant 100 at any
location along its length or width is a function of the cartilage and/or bone
to be replaced.
[0133]
FIG. 8 F is
a lateral view of the implant 100 of FIG. 8A. In this instance
the height of the implant 100 at a first end h1 is
different than the height of the implant at a second end h2. Further
the upper edge 108 can have an overall slope in a downward
direction. However, as illustrated the actual slope of the upper
edge 108 varies along its length and can, in some instances, be a
positive slope. Further the lower edge 110 can have an overall
slope in a downward direction. However, as illustrated the actual slope of
the lower edge 110 varies along its length and can, in some
instances, be a positive slope.
[0134]
FIG.
8 G is a cross-section taken along a sagittal plane in a body showing
the implant 100 implanted within a knee joint 120 such
that the implant 100 lies on the tibial plateau 122 and
the femur 124 rests on the upper surface 102 of
the implant 100. FIG. 8H is a cross-section taken along a
coronal plane in a body showing the implant 100 implanted within
a knee joint 120. As is apparent from this view, the implant 100 is
positioned so that it fits within a superior articular surface 124.
As will be appreciated by those of skill in the art, the articular surface
could be the medial or lateral facet, as needed.
[0135]
FIG. 8 I is
a cross-section along an axial plane of the body showing the implant 100 implanted
within a knee joint 120 showing the view taken from an aerial, or
upper, view. FIG. 8J is a cross-section of an alternate embodiment where
the implant is a bit larger such that it extends closer to the bone medially,
i.e. towards the edge of the tibial plateau, as well as extending anteriorly
and posteriorly.
[0136]
FIG.
8 K is a cross-section of an implant 100 of the
invention according to an alternate embodiment. In this embodiment,
the lower surface 104 further includes a joint anchor 112.
As illustrated in this embodiment, the joint anchor 112 forms a
protrusion, keel or vertical member that extends from the lower
surface 104 of the implant 100 and projects into, for
example, the bone of the joint. Additionally, as shown in FIG.
8L the joint anchor 112 can have a cross-member 114 so
that from a bottom perspective, the joint anchor 112 has the
appearance of a cross or an “x.” As will be appreciated by those of skill in
the art, the joint anchor 112 could take on a variety of other
forms while still accomplishing the same objective of providing increased
stability of the implant 100 in the joint. These forms include,
but are not limited to, pins, bulbs, teeth, balls, etc. Additionally, one or
more joint anchors 112 can be provided as desired.
[0137]
The device can have
two or more components, one essentially mating with the tibial surface and the
other substantially articulating with the femoral component. The two components
can have a flat opposing surface. Alternatively, the opposing surface can be
curved. The curvature can be a reflection of the tibial shape, the femoral
shape including during joint motion, and the meniscal shape and combinations thereof.
FIG. 8 M and N illustrate cross-sections of alternate
embodiments of a dual component implant from a side view and a front view.
[0138]
Turning now to FIG.
9 A-F an implant suitable for providing an opposing joint surface to
the implant of FIG. 8A is shown. This implant corrects a defect on an
inferior surface of the femur (i.e., the portion of the femur that mates with,
e.g., the tibial plateau) and can be used alone, i.e., on the femur, or in
combination with another joint repair device. FIG. 9A shows a perspective
view of the implant 150 having a curved mating
surface 152 and convex joint abutting surface 154.
The joint abutting surface 154 need not form an anatomic or near
anatomic fit with the femur in view of the anchors 156 provided
to facilitate connection of the implant to the bone. In this instance,
the anchors 156 are shown as pegs having notched heads. The
notches facilitate the anchoring process within the bone. However, pegs without
notches can be used as well as pegs with other configurations that facilitate
the anchoring process. Pegs and other portions of the implant can be porous
coated. The implant can be inserted without bone cement or with use of bone
cement. The implant can be designed to abut the subchondral bone, i.e. it can substantially
follow the contour of the subchondral bone. This has the advantage that no bone
needs to be removed other than for the placement of the peg holes thereby
significantly preserving bone stock. As will be appreciated by those of skill
in the art, the multi-component solution illustrated in FIG. 9 for repairing
the hip can be applied to other joints within the body as well.
[0139]
FIG.
9 G and 9 H illustrate an implant 151 suitable
for providing an opposing surface to the implant of FIG. 8A, wherein the
implant is intended to cover both femoral condyles and can optionally oppose
one or more of the implants of FIG. 8A.
[0140]
The arthroplasty
system can be designed to reflect aspects of the tibial shape and/or femoral
shape. Tibial shape and femoral shape can include cartilage and bone or either.
Moreover, the shape of the implant can also include portions or all components
of other articular structures such as the menisci. The menisci are
compressible, in particular during gait or loading. For this reason, the
implant can be designed to incorporate aspects of the meniscal shape accounting
for compression of the menisci during loading or physical activities. For
example, the undersurface of the implant can be designed to match the shape of
the tibial plateau including cartilage or bone or both. The superior surface of
the implant can be a composite of the articular surface of the tibia (in
particular in areas that are not covered by menisci) and the meniscus. Thus,
the outer aspects of the device can be a reflection of meniscal height.
Accounting for compression, this can be, for example, 20%, 40%, 60% or 80% of
uncompressed meniscal height.
[0141]
In some embodiments,
the outer aspect of the device reflecting the meniscal shape can be made of
another, preferably compressible material. If a compressible material is
selected it is preferably designed to substantially match the compressibility
and biomechanical behavior of the meniscus. The entire device can be made of
such a material or non-metallic materials in general.
[0142]
The height and shape
of the menisci can be measured directly on an imaging test. If portions, or
all, of the meniscus are torn, the meniscal height and shape can be derived
from measurements of a contralateral joint or using measurements of other
articular structures that can provide an estimate on meniscal dimensions.
[0143]
In another
embodiment, the superior face of the implant can be shaped according to the
femur. The shape can preferably derived from the movement patterns of the femur
relative to the tibial plateau thereby accounting for variations in femoral
shape and tibiofemoral contact area as the femoral condyle flexes, extends,
rotates, translates and glides on the tibia and menisci.
[0144]
The movement
patterns can be measured using any current or future test know in the art such
as fluoroscopy, MRI, gait analysis and combinations thereof.
[0145]
B. The Hip
[0146]
FIG.
10 A is a side view of the acetabulum 200 of the hip.
The cartilage covered area 202 has an inverted U-shape. The
triradiate cartilage area or acetabular fossa 204 is located
within the cartilage covered area. FIG. 10B is a rotated view of
the proximal femur 210. The cartilage covered area 202 and
the fovea capitis 206 are also shown.
[0147]
Turning now to
implants suitable for the hip joint, FIG. 10 C is a cross-section of
an implant for a hip joint 220. The radius r of this implant is
substantially constant when taken at any point along its length. The radius of
the implant can be selected to approximate the radius of the femoral head that
the implant is intended to correct and can be measured to an interior surface
of the implant 220 that engages the femoral head. Alternatively,
the radius of the implant can be selected to approximate the radius of the
acetabulum or a combination thereof. The radius of the interior
surface 222 of the implant faces the femur and can also match the
radius of the femur or be similar to the radius of the acetabulum; the radius
of the implant surface facing the acetabulum can also match that of
the acetabulum 224 or be similar to that of the femur.
[0148]
A person of skill in
the art will appreciate that the natural geometry of the acetabulum typically
is aspherical, varying slightly from a true spherical shape. The radius of the
implant adjusts, as necessary, to the changing radius of the acetabulum to
provide a better fit. Thus, implants can be spherical or aspherical in radius
on either or both of the superior and/or inferior surface.
[0149]
FIG. 10 D is
a cross-section of an implant suitable for the hip similar to that seen in FIG.
10C, featuring a rounded margin 226. A round margin 226 can
be advantageous because it tends to avoid locking of the implant when in use as
well as minimizing any pain that might be associated with the implant.
[0150]
FIG.
11 A is a cross-section of an implant 220 suitable for
the hip similar to that shown in FIG. 10C with a nub 230 is
provided that extends into the fovea capitis of the femoral head 240 on
its interior surface 222. The member 230 can be made
of the same material as the implant 220, or a material different from
the remainder of the implant. The advantage of an implant having
a nub 230 for engaging the fovea capitis is that
the nub 230 can function to constrain movement of
the implant 220 relative to the femoral head (shown in FIG.
10B). As will be appreciated by those of skill in the art, the nub 230 can
take a variety of configurations while still accomplishing the same effect when
engaging the fovea capitis upon implantation. A variety of plan views are shown
that provide for an implant that is hemispherical, partially hemispherical, or
in the form of a rail. Additional shapes will be apparent to those of skill in
the art. Additionally, the edges of the implant can be rounded, beveled or
whatever dimension that facilitates the operation of the implant. FIG.
11B-E illustrate alternative embodiments of the implant shown in FIG. 11A,
wherein the implant is hemispherical, partially hemispherical, rail and spoke.
[0151]
FIG.
12 A is a cross-section of an implant 220 suitable for
the hip with a ledge 232 that extends into the acetabular
fossa 204 on its exterior surface 224. The ledge 232 can
be made of the same or a different material as the remainder of
the implant 220. The ledge 232 can be used to
constrain movement of the implant relative to the acetabular fossa. As will be
appreciated by those of skill in the art, the ledge 232 can take
a variety of configurations while still accomplishing the same effect when engaging
the acetabular fossa. A variety of plan views are shown that provide for an
implant that is hemispherical, partially hemispherical, or in the form of a
rail or four-prong cap. Additional shapes will be apparent to those of skill in
the art. Additionally, the edges of the implant can be rounded, beveled or
whatever dimension that facilitates the operation of the implant. FIG.
12B-E illustrate alternative embodiments of the implant shown in FIG. 12A,
wherein the implant is hemispherical, partially hemispherical, rail and spoke.
[0152]
FIG.
13 A is a cross-section of a dual component “mobile
bearing” implant 221 with a variety of plan views. The implant
has a first component 230 and a second component 231.
The first component fits within the second component and has two smooth
surfaces. The second component engages the outer surface of the first component
and also has two smooth surfaces. A variety of configurations in plan is
possible without departing from the scope of the invention. For example, each
component can be hemispherical. One component can be hemispherical while the
other one takes on a shape that is a part-hemisphere, a shorter hemisphere, a
rail, or a four-prong dome. FIG. 13B-F illustrate a variety of alternative
embodiments of the implant shown in FIG. 13A, wherein the implant has at least
one component that is hemispherical, partially hemispherical, rail and spoke.
[0153]
FIG.
13 G-J are cross-sectional views of a dual component “mobile bearing”
implant. The implant has a first component and a second component. The first
component fits within the second component. The second component engages the
outer surface of the first component. As shown herein a nub is provided on the
second component that fits within an indentation on the first component. As
will be appreciated by those of skill in the art, although not shown, the nub
could be on the first component and fit within a well on the second component
without departing from the scope of the invention. Additional anchoring
mechanisms either on the first component, second component, or both are also
possible, as shown. A variety of configurations in plan is possible, although
not shown, without departing from the scope of the invention. For example, each
component can be hemispherical. One component can be hemispherical while the
other one takes on a shape that is a part-hemisphere, a shorter hemisphere, a
rail, or a four-prong dome.
[0154]
FIG.
14 A is a cross-section of another dual component “mobile
bearing” implant 240 with a nub 246 for extending
into the fovea capitis 206 of the femoral head. The dual
component implant 240 has a first component 242 and
a second component 244. A nub 246 is provided on
the second component 244. As described above with respect to FIG.
11C, the nub 246 can be used to constrain movement of
the second component 244 of the implant 240 relative
to the femoral head. The first component 242 facing the
acetabulum can move freely relative to the second component 244 facing
the femoral head. As will be appreciated by those of skill in the art, the dual
component implant can be configured such that the surface of the first
component 243 that engages the surface of the second
component 245 have the same length, or substantially the same length.
Thus creating mating components that fit substantially within one another.
Alternatively, the components can be configured such that one component is
shorter than another component as shown in FIG. 14B and FIG. 14C. FIG. 15A is
a cross-section of another dual component “mobile bearing” implant 240 with
a ledge 248 extending into the acetabular fossa. The dual
component implant 240 has a first component 242 and
a second component 244. A ledge 248 is provided on
the first component 242. The ledge 248 can be used to
constrain movement of the first component 242 of
the implant 240 relative to the acetabulum. The second
component 244 facing the femoral head can move freely relative to
the first component 242 facing the acetabulum. As described
above with respect to FIG. 13A, the implant shown in FIG. 15A can also be
configured such that one component is shorter than another component as shown
in FIG. 15B and 15 C.
[0155]
FIG.
16 A is a cross-section of a triple component “mobile
bearing” implant 250. The first component 252 facing
the acetabulum has a nub 253 extending into the acetabular
fossa 204. As discussed above, the nub 253 can be used to
constrain movement of the implant 250 relative to the
acetabulum. The second component 254 facing the femoral head has
a ledge 255 extending into the fovea capitis 206. As
discussed above with respect to the single and dual member implants,
the ledge 255 can be used to constrain movement of
the second component 254 of the implant 250 relative
to the femoral head. The third component 256 is interposed
between the two other components and can move freely between them. As will be
appreciated by those of skill in the art, the third component 256 can
be interposed between the first 252 and second 254 components
such that its length is shorter than either the first 252 or
second 254 components (as shown in FIG. 16B) or longer than either of
the first 252 or second 254 components (as shown in FIG.
16C and 16 D). Similarly, it would be possible for the length of
the third component to be longer than either of the first 252 or
second 254 components.
[0156]
FIG.
17 A is a cross-section of another dual component “mobile
bearing” implant 240 similar to those shown above. In this
embodiment, anchors are provided to anchor the first component 242 to
the acetabular fossa 204. The anchors shown are in the form of one or
more pins 262. The component facing the acetabulum is fixed to the
acetabulum using two substantially parallel pegs. The second
component 244 facing the femoral head can move freely on
the first component 242 facing the acetabulum. As with the
previous embodiments, the length of the first component 242 relative
to the second component 244 can vary. FIG. 17B and 17 C show
alternate cross-sectional views where a first component is larger that a second
component, and vice versa. As with the previous embodiments, a variety of
configurations in plan is possible without departing from the scope of the
invention. For example, each component can be hemispherical. One component can
be hemispherical while the other one takes on a shape that is a
part-hemisphere, a shorter hemisphere, a rail, or a four-prong dome.
[0157]
FIG.
18 A is a cross-section of another dual component “mobile
bearing” implant 240 with an anchor extending into
the acetabular fossa 204. The anchor facing the acetabulum is in the
form of a protrusion having one or more fins 264. The second
component 244 facing the femoral head can move freely on the first
component 242 facing the acetabulum. FIG. 18B is a view of the
implant of FIG. 18A from the top showing four fins (264, 264′, 264″, 264′″)
on top of the member extending into the acetabular fossa on top of the
acetabular component. The fins can be sharp or substantially sharp as shown or
can have rounded edges.
[0158]
FIG.
19 A is a cross-section of another dual component “mobile
bearing” implant 240 with an anchor 266 capable
of extending into the fovea capitis 206 of the femoral head. In the
embodiment shown, the second component 244 facing the femoral
head is fixed to the femoral head using one or more substantially parallel pegs
(shown as 268, 268′b. The first component 242 faces
the acetabulum, as shown in previous embodiments, and can move freely on the
component facing the femoral head.
[0159]
FIG.
19 B is a cross-section of another dual component implant 240.
In this embodiment, the dual component 240 is fixed. As
illustrated herein, the femoral component is attached to the femoral head using
3 pegs 266 or other attachment mechanisms. The number of pegs
can be greater or less than 3, as desired. Preferably, the subchondral bone
remains intact with this design except for the entry point of the pegs. The
acetabular component is attached to the acetabulum using fins 264 or
similar attachment means such as pegs (shown in FIG. 17A). The attachment
mechanism can be molded to the acetabular fossa with members extending into the
bone. The subchondral bone preferably also remains intact except for the entry
area for the attachment means.
[0160]
FIG.
20 A is a cross-section of an implant 470 with varying
radii (r1, r2, r3) and thickness (t1, t2, t3) for a hip joint; where r1≠r2≠r3 and
thickness t1≠t2≠t3. As will be appreciated by those of skill in the art, three
measurements of radii and thickness have been taken to illustrate the point,
but more or less measurements can be used without departing from the scope of
the invention. Additionally, other combinations of radii and thicknesses can be
employed such that, for example, r1=r2≠r3, r1≠r2=r3, t1=t2≠t3 and t1≠2=t3.
Other combinations will be apparent to those of skill in the art. As
illustrated in FIG. 20A, the central portion c that has a thickness t that is
thicker relative to one or both peripheral portions p1, p2.
[0161]
FIG.
20 B is a cross-section of an alternate implant 470 with
varying radii and thickness for a hip joint. In this embodiment, the central
portion c has a thickness tc that is thinner relative to one or more
thicknesses t1, t2 of the peripheral portions (p1, p2)
[0162]
FIG.
20 C is a cross-section of an alternate implant 470 with
varying radii and thickness for a hip joint. In this embodiment, the central
portion c has a thickness tc that is thinner relative to the thickness t1 of
a first peripheral end p1, and thicker relative to the thickness t2 of a
second peripheral end p2 of the peripheral portions.
[0163]
FIG.
20 D is a cross-section of an alternate implant 470 for
a hip joint with one or more lips or anchoring extensions extend inferiorly Ii and/or
superiorly Is. The lips are designed to extend beyond the articular surface,
e.g. into non-cartilaginous areas. It can substantially conform to the
surrounding, periarticular anatomy. The lips can provide additional
stabilization. This design can be combined with dual and triple component and
“mobile-bearing” designs.
[0164]
As will be
appreciated by those of skill in the art, the three-dimensional shape of the
implants shown in FIGS. 10-20 can be semicircular (i.e., 180°) in one
or more dimension, but need not be. Where the implant is semicircular in all
dimensions, the implant forms a hemisphere (i.e., half of a sphere obtained by
cutting it by a plane passing through its center). Where the implant is
semicircular in some, but not all dimension, its shape will not be
hemispherical. The shape can be aspherical on either or both of the superior
and inferior surfaces to accommodate the acetabulum. Further, where there is more
than one component, a combination of three dimensional shapes can be employed.
For example, a first component can be hemispherical, while a second component
is not, and so on.
[0165]
Additionally, while
these implants have been shown having from one to three components, it will be
appreciated, that each component can be further modified into a plurality of
components that engage with one another without departing from the scope of the
invention.
[0166]
It will further be
appreciated by those of skill in the art that the design considerations taught
in FIGS. 10-20 can be employed in designing implants for other
joints, such as the knee, ankle, shoulder, elbow, and wrist. To avoid obscuring
the invention, all possible configurations of the implants taught herein have
not been shown.
[0167]
C. The Shoulder
[0168]
FIG.
21 A is a frontal view of the osseous structures in the shoulder
joint 300 such as the clavicle 302, scapula 304, glenoid
fossa 306, acromion 308, coracoid process 310 and humerus 312.
The cartilage covered areas 314, 316 are indicated by the
oblique lines.
[0169]
FIG.
21 B is a view of an arthroplasty device 320 placed
between the humeral head 313 and the glenoid fossa 306.
The arthroplasty device 320 can have similar design features as
the ones shown in FIGS. 4A-4 R, e.g. a plurality of components, mobile
bearing designs, attached and unattached designs, designs with varying
thickness and curvatures, designs conforming to the humeral head 313 or glenoid
fossa 306 or both, designs conforming to the articular cartilage
and/or subchondral bone, designs with lips or members for stabilization
purposes.
[0170]
FIG.
21 C is an oblique frontal cross-sectional view of
an arthroplasty device 320 with a humeral contacting surface 322 that
conforms at least partially to the shape of the humeral head 313 and
a glenoid contacting surface 324 that conforms at least
partially to the shape of the glenoid fossa 306.
[0171]
FIG.
21 D is an axial cross-sectional view of an arthroplasty device 520 with
a humeral contacting surface 322 that conforms to the shape of
the humeral head and a glenoid contacting surface 324 that
conforms to the shape of the glenoid fossa 306.
[0172]
FIG.
21 E is an oblique frontal view of the shoulder joint illustrating
the articular cartilage 316 and the superior and
inferior glenoid labrum 306′, 306″, respectively. FIG.
21F is an axial view of the shoulder joint illustrating the articular
cartilage 316 and the anterior and posterior glenoid
labrum 307′, 307″, respectively.
[0173]
FIG.
21 G is an oblique frontal cross-sectional view of
an arthroplasty device 320 with the humeral
contacting surface 322 that conforms to the shape of
the humeral head 313 and a glenoid contacting surface 324 that
conforms to the shape of the glenoid 306 and the glenoid labrum (306′, 306′).
FIG. 21H is an axial cross-sectional view of an arthroplasty
device 320 shown in FIG. 21G. As shown above, a humeral
contacting surface 322 is provided that conforms to the shape of
the humeral head 313 and a glenoid contacting surface 324 is
provided that conforms to the shape of the glenoid 306 and the
glenoid labrum.
[0174]
FIG.
21 I is an oblique frontal cross-sectional view of an alternate
embodiment of an arthroplasty device 340 with the humeral
contacting surface 342 that conforms to the shape of
the humeral head 313 and a glenoid contacting surface 344 that
conforms substantially to the shape of the glenoid 306. One or more
protrusions or lips 346, 346′ can be provided that extend
superiorly and/or inferiorly. The lips can be configured to provide
stabilization over the glenoid. FIG. 21J is an axial cross-sectional view
of the arthroplasty device 340 shown in FIG. 21I with the
humeral contacting surface 342 that conforms to the shape of
the humeral head 313 and the glenoid contacting
surface 344 that conforms substantially to the shape of the
glenoid 306. One or more lips 346″, 346′″ can be provided
that extend anteriorly and/or posteriorly providing stabilization over the
glenoid 306.
[0175]
FIG.
21 K is an oblique frontal cross-sectional view of a dual component,
“mobile-bearing” arthroplasty device 350 with the humeral
contacting surface 354 of a first component 351 that
conforms to at least a portion of the humeral head and a glenoid
contacting surface 354 of a second component 353 that
conforms to at least a portion of the shape of the glenoid. As will be
appreciated by those of skill in the art, the radius (radii) of the two
articulating implant surfaces can be selected to match or substantially match
that of the humerus or the glenoid or both. Further the implant can have a
contacting surface that conforms with the humerus or glenoid either
substantially, or as much as necessary to achieve the desired correction and
functional effect. Moreover, the center of rotation of the two articulating
implant surfaces 356, 358 can be selected to match substantially
the center of rotation of the humeral head. As will be appreciated by those of
skill in the art, the two articulating implant surfaces 356,358 can
have any shape including a flat surface.
[0176]
FIG.
21 L is an axial cross-sectional view of a dual component,
“mobile-bearing” arthroplasty device shown in FIG. 21K. The humeral
contacting surface 352 is configured as shown in this embodiment
so that it conforms substantially to the shape of the humeral head 313 and
the glenoid contacting surface 354 is configured in this
embodiment so that it conforms substantially to the shape of the glenoid 306.
The radius (radii) of the two articulating implant surfaces can be selected to
match the surfaces of the humerus, the glenoid, or both. Moreover, the center
of rotation of the two articulating implant surfaces can be selected to match
substantially the center of rotation of the humeral head. FIG. 21M is an
alternate embodiment showing the implant with an indentation on one component
and a ball on a second component. The indent and ball configuration can be
reversed such that it is on the opposing surface without departing from the
scope of the invention. As will be appreciate the ball and socket arrangement
shown will facilitate the movement of the implant components relative to each
other but can assist in preventing undesirable movement of the components in
operation.
[0177]
FIG. 21 N is
an oblique frontal cross-sectional view of an alternate embodiment of a dual
component, “mobile-bearing” arthroplasty implant 360.
The implant 360 has a first component 362 and
a second component 364. The glenoid component 364 is
configured to have two surfaces. The first surface 363 is
configured to articulate relative to the first component 362.
The second surface 363 is configured to mate with the
glenoid 306. The second, or glenoid, component 364 is
attached to the glenoid using one or more anchors 365. The anchor 365 can
be in the form of pegs or fins or other suitable configurations to achieve the
desired result of anchoring the glenoid component 364 to the
glenoid. These pegs or fins can be cemented, porous coated, or both. Similarly,
the glenoid contacting surface 363 of the component 362 can
be cemented, porous coated, or both. Preferably, only the anchor 365 extends
into the subchondral bone.
[0178]
FIG.
21 O is an oblique frontal cross-sectional view of an alternate
embodiment of a dual component, “mobile-bearing” arthroplasty device 370.
The humeral contacting component 372 is attached to
the humeral head 312 using an attachment mechanism such as pegs
or fins or, as illustrated in this example, spikes 373. These pegs, fins,
teeth or spikes can be cemented, porous coated, or both. Similarly, the
undersurface of the humeral component can be cemented or porous coated or both.
Preferably, only the attachment mechanism itself (i.e., the pegs, fins or
spikes) can extend through the subchondral bone. The pegs, fins, teeth or
spikes can be pyramidal, conical, triangular, spherical, tubular, or
protrusions of any kind and can be in a random configuration on the surface or
an organized configuration (e.g., rows). As illustrated herein there
is articular cartilage 374 on the glenoid side of the joint.
The implant 370 can be designed to conform to the articular
cartilage 374 or the subchondral bone, or both. As shown in FIG.
21P and 21 Q the fins or spikes can be alternating lengths
and can be configured such they the fins are parallel to each other.
[0179]
In another
embodiment, the implant can be adapted to soft-tissue damage. For example, in
the event of a rotator cuff tear, the implant can have an extension covering
portions or all of the superior aspect of the humeral head. In this manner,
superior migration of the humeral head as a result of the tear of the rotator
cuff cannot lead to pathologic articulation of the humeral head with the
acromioclavicular joint with resultant pain and disability. Instead, the
superior aspect of the humeral head can articulate with extended member of the
implant thereby avoid eburnation of the AC joint.
[0180]
D. The Elbow
[0181]
FIG. 22 is an
oblique longitudinal view through the elbow joint 600 demonstrating
the distal humerus 602, the olecranon 604 and
the radial head 606. The cartilaginous surfaces are seen 603, 605, 607,
respectively. An arthroplasty device 620 is illustrated
interposed between the distal humerus and the articulating surfaces on
the ulna 608 and radius 610. The arthroplasty
device 620 can have similar design features as those illustrated with
respect to the devices shown in FIGS. 10-20, e.g. single, dual, triple
component; mobile bearing designs; attached and unattached designs; designs
with varying thickness and curvatures; designs conforming to the humerus or
ulna or radius or combinations thereof; designs conforming to the articular
cartilage and/or subchondral bone, designs with lips or members for
stabilization purposes. However, to avoid obscuring the invention, each
possible permutation of design consideration taught in this application has not
been illustrated for this joint.
[0182]
E. The Wrist
[0183]
FIG.
23 A is a longitudinal view through the wrist joint 700 demonstrating
the distal radius 702, the ulna 704 and several of the
carpal bones which form a carpal row 706 (e.g. scaphoid, lunate,
triquetral, capitate and hamate). An arthroplasty device 720 is
illustrated interposed between the distal radius 702, the distal
ulna 704 and the articulating surfaces of the proximal carpal
row 706′, 706″, 706′″. The arthroplasty device 720 conforms
to the shape of the distal radius 702, the proximal carpal
row 706, and, in this example, the triangular fibrocartilage (dotted
lines) 708.
[0184]
As will be
appreciated by those of skill in the art, the arthroplasty device 720 can
have design features similar to those described with relation to the devices
shown in FIGS. 10-20, e.g. single, dual, triple component; mobile bearing
designs; attached (e.g. to the distal radius) and unattached designs; designs
with varying thickness and curvatures; designs conforming to the radius or ulna
or carpals or combinations thereof; designs conforming to the articular
cartilage and/or subchondral bone and also to other articular structures such
as the triangular fibrocartilage; designs with lips or members for
stabilization purposes.
[0185]
FIG.
23 B is a longitudinal view through the wrist joint 700 demonstrating
the distal radius 702, the ulna 704 and several of
the carpal bones 706. An arthroplasty device 720 is
illustrated interposed between the distal radius 702, the distal
ulna 704 and the articulating surfaces 706′, 706″, 706′″
of the proximal carpal row 706. The arthroplasty device 720 is
configured such that it conforms to at least a portion of the shape of
the distal radius 702, the distal ulna 704, and the
proximal carpal row 706.
[0186]
FIG.
23 C is a longitudinal view through the wrist joint 700 again
demonstrating the distal radius 702, the ulna 704 and
several of the carpal bones 706. An arthroplasty device 730 is
shown interposed between the distal radius 702, the distal
ulna 704 and the articulating surfaces 706′, 706″, 706′″
of the proximal carpal row 706. The arthroplasty device 730 shown
conforms substantially to the shape of the distal radius 702, the
proximal carpal row 706 and the distal ulna 704 including
the ulnar styloid 710. A lip 732 is seen extending
along the medial aspect of the distal radius and the lateral aspect of
the distal ulna 704 including the ulnar styloid 710;
this can provide stabilization of the implant relative to these bones. One
or more lips 732, or other suitably configured protrusions, can
extend towards the dorsal or palmar aspect of any of the bones of the joint.
[0187]
FIG.
23 D is a longitudinal view of a dual component,
“mobile-bearing” arthroplasty device 740. The device 740 has
a first component 742 and a second component 744. Each
component has a surface that articulates with a surface of the other
component, 743, 745. The radii of the two articulating implant
surfaces can be selected to match that of the radius 702 or
the ulna 704 or the carpal bones 706 or combinations
thereof. Moreover, the center of rotation of the two articulating implant
surfaces can be selected to match or approximate the center of rotation of the
joint 700. As will be appreciated by those of skill in the art, the two
articulating implant surfaces 743, 745 can have any shape that
facilitates the functioning of the joint, including a flat surface. Note
the lips 746, 748 of the proximal component extending
medially and laterally. Lips can also extend towards the dorsal or palmar
aspect.
[0188]
FIG.
23 E is a longitudinal view of another dual component,
“mobile-bearing” arthroplasty device 750, in this case without lips.
The device 750 has a first component 752 and
a second component 754. Each component has a surface that articulates
with a surface of the other component, 753, 755. As evident from the
cross-sectional view, the length of the first component's
articulating surface 753 is longer than the length of the second
component's articulating surface 755.
[0189]
FIG.
23 F is a longitudinal view of a dual component, “mobile-bearing”
arthroplasty device 760. As depicted, the first component 762 facing
the radius and ulna has been attached to these bones using an attachment
mechanism or anchor 766. Suitable anchors 766 include
pegs, as shown in this example, spikes and/or fins, to name a few. As will be
appreciated by those of skill in the art, the attachment of the device 760 can
be limited to attachment to one bone only (e.g. the ulna or the radius).
[0190]
F. The Finger
[0191]
FIG. 24 is a
sagittal view through a finger 800. An arthroplasty device 820 is
illustrated such that it is interposed between the metacarpal head 802 and
the base of the proximal phalanx 804. The arthroplasty
device 820 conforms to the shape of the metacarpal head 802 on
one side 822 and the base of the proximal phalanx 804 on
an opposing side 824. The arthroplasty device 820 can
have similar design features as the ones seen in FIGS. 10-20, e.g. single,
dual, triple component, mobile bearing designs, attached (e.g. to the
metacarpal head or the base of the phalanx) and unattached designs, designs
with varying thickness and curvatures, designs conforming to the proximal or
the distal articular surface or combinations thereof, designs conforming to the
articular cartilage and/or subchondral bone and also to other articular
structures, designs with lips or members for stabilization purposes. Similar
designs are applicable to the hind, mid and forefoot including the toes.
[0192]
G. The Ankle
[0193]
FIG.
25 A is a sagittal view through the ankle joint 900 demonstrating
the distal tibia 902, the talus 904 and calcaneus 906 and
the other bones. The cartilaginous surfaces are also shown.
An arthroplasty device 920 is illustrated interposed between
the distal tibia 902 and the talar dome 904′, In this
example, the arthroplasty system 920 conforms to the shape of
the talus 904. As will be appreciated by those of skill in the art,
and discussed previously, the device can conform to the shape of the cartilage
or the subchondral bone or both. The arthroplasty device 920 can
have similar design features as the devices illustrated in FIGS. 10-20 and
discussed above, e.g. single, dual, triple component, mobile bearing designs,
attached and unattached designs, designs with varying thickness and curvatures,
designs conforming to the tibia or talus or fibula or combinations thereof,
designs conforming to the articular cartilage and/or subchondral bone, designs
with lips or members for stabilization purposes.
[0194]
FIG.
25 B is a coronal view through the ankle joint 900 illustrating
the distal tibia 902, the distal fibula 908 and
the talus 904. An arthroplasty device 930 is
illustrated interposed between the distal tibia 902 and
the talar dome 904′. In this example, the arthroplasty
system 930 is shown conforming to the shape of the talus 904.
[0195]
FIG.
25 C is a sagittal view through the ankle joint 900 illustrating
the distal tibia 902, the talus 904 and calcaneus 906 and
the other bones. The cartilaginous surfaces are also shown.
An arthroplasty device 940 is depicted interposed between
the distal tibia 902 and the talar dome 904′, In this
example, the inferior surface of the arthroplasty system 942 conforms
substantially to the shape of the talus 904. The superior
surface 944 conforms substantially to the shape of the distal
tibia 902 and fibula (908, not shown). A lip 946 is
shown on the inferior surface 942 that engages
the talus 904.
[0196]
FIG.
25 D is a coronal view through the ankle joint 900 illustrating
the distal tibia 902, the distal fibula 908 and
the talus 904. An arthroplasty device 950 is shown
interposed between the distal tibia 902 and the talar
dome 904′, In this example, the inferior surface 952 of the
arthroplasty system conforms to the shape of the talus 904. The superior
surface 954 conforms to the shape of the distal tibia 902 and fibula 908.
[0197]
H. The Toe
[0198]
FIG. 26 is a
sagittal view through a toe 1000. An arthroplasty device 1020 is
illustrated interposed between the metatarsal head 1002 and the
base of the proximal phalanx 1004. The arthroplasty device 1020 illustrated
conforms to the shape of the metatarsal head on a first surface 1022 and
the base of the proximal phalanx on a second surface 1024. As will be
appreciated by those of skill in the art, the arthroplasty device can have
similar design features as the ones seen in FIGS. 10-20, e.g. single,
dual, triple component, mobile bearing designs, attached (e.g. to the
metatarsal head or the base of the phalanx) and unattached designs, designs
with varying thickness and curvatures, designs conforming to the proximal or
the distal articular surface or combinations thereof, designs conforming to the
articular cartilage and/or subchondral bone and also to other articular
structures, designs with lips or members for stabilization purposes. Similar
designs are applicable to the hind, mid and forefoot.
[0199]
D. Device
Manufacture, Composition and Properties
[0200]
The devices
described above, or any device manufactured according to the teachings of this
invention, can be prepared from a variety of suitable materials known in the
art
[0201]
A wide variety of
materials find use in the practice of the present invention, including, but not
limited to, plastics, metals, ceramics, biological materials (e.g., collagen or
other extracellular matrix materials), hydroxyapatite, cells (e.g., stem cells,
chondrocyte cells or the like), or combinations thereof. Based on the
information (e.g., measurements) obtained regarding the defect and/or the
articular surface and/or the subchondral bone, a suitable material can be
selected. Further, using one or more of these techniques described herein, a
cartilage replacement or regenerating material having a curvature that can fit
into a particular cartilage defect, can follow the contour and shape of the
articular surface, and can match the thickness of the surrounding cartilage can
be formed. Moreover, using one or more of these techniques described herein, an
articular device can be shaped that can fit into a joint space and that can
follow the contour and shape of the articular surface or other articular
structures. The material can include any combination of materials, and
preferably includes at least one substantially non-pliable material.
[0202]
Additionally, the
material can have a gradient of hardness. Thus, for example, the gradient of
hardness can decrease from the center of the device to an outer edge. Thus
producing a device that has overall firmness, but which has a bit of give to
the surface along some or all of the outside surfaces. Providing an exterior
surface made of material with some give could enhance the implant's ability to
mate with the joint. Alternatively, in some scenarios a device can be
manufactured where the exterior surface has a Shore hardness value higher than
its interior sections.
[0203]
The exterior
hardness of the devices will be suitable for the implant to perform within the
joint. Suitable hardnesses will be obvious to those of skill in the art and can
comprise a range. Typically, harnesses are discussed in terms of the Shore
hardness scale and can range from that common for engineering grade plastics to
hardened steel and titanium, and preferably on the portion of the Rockwell
hardness scale typical of steels, hard plastics and ceramic materials. From the
high hardness desired of the device, it is readily apparent that the device
functions in a manner completely different from those of the prior art. The
purpose of the device of the subject invention is to achieve a span-like effect
to bridge the defective areas. However, in a composite variation, any single
component (like a bioactive material component described below) can be softer
than the supporting material.
[0204]
Currently, joint
repair systems, including devices, employ metal and/or polymeric materials.
See, e.g., U.S. Pat. No. 6,203,576 to Afriat, et al., issued Mar. 20, 2001;
U.S. Pat. No. 6,206,927 to Fell, et al., issued Mar. 27, 2001; and U.S. Pat.
No. 6,322,588 to Ogle, et al.; issued Nov. 27, 2001 and references cited
therein. Similarly, a wide-variety of metals can find use in the practice of
the present invention, and can be selected based on any criteria, for example,
based on resiliency to impart a desired degree of rigidity. Non-limiting
examples of suitable metals include silver, gold, platinum, palladium, iridium,
copper, tin, lead, antimony, bismuth, zinc, titanium, cobalt, stainless steel,
nickel, iron alloys, cobalt alloys, such as Elgiloy®, a cobalt-chromium-nickel
alloy, and MP35N, a nickel-cobalt-chromium-molybdenum alloy, and Nitinol™, a
nickel-titanium alloy, aluminum, manganese, iron, tantalum, other metals that
can slowly form polyvalent metal ions, for example to inhibit calcification of
implanted substrates in contact with a patient's bodily fluids or tissues, and
combinations thereof.
[0205]
Suitable synthetic
polymers include, without limitation, polyamides (e.g., nylon), polyesters,
polystyrenes, polyacrylates, vinyl polymers (e.g., polyethylene,
polytetrafluoroethylene, polypropylene and polyvinyl chloride), polycarbonates,
polyurethanes, poly dimethyl siloxanes, cellulose acetates, polymethyl
methacrylates, polyether ether ketones, polyether ketone ketone, ethylene vinyl
acetates, polysulfones, nitrocelluloses, similar copolymers and mixtures
thereof. Bioresorbable synthetic polymers can also be used such as dextran,
hydroxyethyl starch, derivatives of gelatin, polyvinylpyrrolidone, polyvinyl
alcohol, poly[N-(2-hydroxypropyl) methacrylamide], poly(hydroxy acids),
poly(epsilon-caprolactone), polylactic acid, polyglycolic acid, poly(dimethyl glycolic
acid), poly(hydroxy butyrate), and similar copolymers can also be used.
[0206]
The polymers can be
prepared by any of a variety of approaches including conventional polymer
processing methods. Preferred approaches include, for example, injection molding,
which is suitable for the production of polymer components with significant
structural features, and rapid prototyping approaches, such as reaction
injection molding and stereo-lithography. The substrate can be textured or made
porous by either physical abrasion or chemical alteration to facilitate
incorporation of the metal coating.
[0207]
The polymer can be
injected into a mold reflecting aspects of the articular surface(s) or other
articular structures.
[0208]
More than one metal
and/or polymer can be used in combination with each other. And liquid metals
can be used as well. For example, one or more metal-containing substrates can
be coated with polymers in one or more regions or, alternatively, one or more
polymer-containing substrate can be coated in one or more regions with one or
more metals.
[0209]
The device or parts
thereof can be porous or porous coated. The porous surface components can be
made of various materials including metals, ceramics, and polymers. These
surface components can, in turn, be secured by various means to a multitude of
structural cores formed of various metals. Suitable porous coatings include,
but are not limited to, metal, ceramic, polymeric (e.g., biologically neutral
elastomers such as silicone rubber, polyethylene terephthalate and/or
combinations thereof) or combinations thereof. See, e.g., U.S. Pat. No.
3,605,123 to Hahn, issued Sep. 20, 1971; U.S. Pat. No. 3,808,606 to Tronzo,
issued Apr. 23, 1974; U.S. Pat. No. 3,843,975 to Tronzo issued Oct. 29, 1974;
U.S. Pat. No. 3,314,420 to Smith; U.S. Pat. No. 3,987,499 to Scharchach, issued
Oct. 26, 1976; and German Offenlegungsschrift 2,306,552. There can be more than
one coating layer and the layers can have the same or different porosities.
See, e.g., U.S. Pat. No. 3,938,198 to Kahn, et al., issued Feb. 17, 1976.
[0210]
The coating can be
applied by surrounding a core with powdered polymer and heating until cured to
form a coating with an internal network of interconnected pores. The tortuosity
of the pores (e.g., a measure of length to diameter of the paths through the
pores) can be important in evaluating the probable success of such a coating in
use on a prosthetic device. See, also, U.S. Pat. No. 4,213,816 to Morris,
issued Jul. 22, 1980. The porous coating can be applied in the form of a powder
and the article as a whole subjected to an elevated temperature that bonds the
powder to the substrate. Selection of suitable polymers and/or powder coatings
can be determined in view of the teachings and references cited herein, for
example based on the melt index of each.
[0211]
Any of the devices
described herein can also include one or more biological materials, either
alone or in combination with non-biological materials. Non-limiting examples of
biological materials include cells (e.g., fetal cartilage cells), biological
polymers (e.g., collagen, elastin, silk, keratin, gelatin, polyamino acids, cat
gut sutures, polysaccharides such as cellulose and starch), autografts,
allografts, xenografts, etc. See, U.S. Pat. No. 5,478,739 to Slivka, et al.,
issued Dec. 26, 1995; U.S. Pat. No. 5,842,477 to Naughton, et al., issued Dec.
1, 1998; U.S. Pat. No. 6,283,980 to Vibe-Hansen, et al., issued Sep. 4, 2001;
and U.S. Pat. No. 6,365,405 to Salzmann, et al. issued Feb. 4, 2002.
[0212]
In certain
embodiments, the device can include one or more separate (but preferably
engageable) components. For example, a two-piece device can include two
components, where each component includes a mating surface. The two components
can be interlocking. When mated with one another the contoured faces oppose
each other and form a device that fits within the defect intended to correct
and provides a joint surface that mimics or replicates a natural joint surface.
Any suitable interlocking mechanism can be used, including a slideable (e.g.,
keyway) system; an interlocking clasp; a ball and keyway interlocking system; a
groove and flange system; etc. In some embodiments, the surfaces of the
components that are engageable are curved. The curvature can be a reflection of
one or more articular structures.
[0213]
In other
embodiments, the configuration of the devices changes upon deployment into the
joint. Thus, the devices can be designed in an initial configuration. Upon
deployment, the devices can assume a subsequent configuration that is different
from the initial configuration. For example, the devices can be
multiple-component devices that, in a first configuration, has a small profile
or small three-dimensional shape. Upon deployment the surgeon allows (or causes)
the device to assume a second configuration, which can have a greater profile
or overall three-dimensional shape. The device can be self-forming into its
secondary configuration or, alternatively, can be manipulated, for example by
mechanical means (e.g., unfolding the device or sliding the components of the
device relative to each other so that they assume the larger second
configuration). One advantage of such embodiments is that smaller incisions are
required. The device can, for example, be deployed arthroscopically in this
manner. Thus, assuming the subsequent configuration can be automatic,
semi-automatic, or manual.
[0214]
The methods and
compositions described herein can be used to replace only a portion of the
articular surface, for example, an area of diseased cartilage or lost cartilage
on the articular surface. In these systems, the articular surface repair system
can be designed to replace only the area of diseased or lost cartilage or it
can extend beyond the area of diseased or lost cartilage, e.g., 3 or 5 mm into
normal adjacent cartilage. In certain embodiments, the prosthesis replaces less
than about 70% to 80% (or any value therebetween) of the articular surface
(e.g., any given articular surface such as a single femoral condyle, etc.),
preferably, less than about 50% to 70% (or any value therebetween), more
preferably, less than about 30% to 50% (or any value therebetween), more
preferably less than about 20% to 30% (or any value therebetween), even more
preferably less than about 20% of the articular surface.
[0215]
E. Alternate
Attachment Mechanisms
[0216]
As will be
appreciated by those of skill in the art, a variety of attachment mechanisms
can be provided to attach the implants within a target joint. For example an
attachment mechanism can be ridges, pegs, pins, cross-members, and other
protrusions that engage the implant mating surface. These protrusions or
mechanisms can have a variety of shapes and cross-sections including,
pyramidal, triangular, conical, spherical, cylindrical, circular, etc. A single
attachment mechanism can be used or a plurality of mechanisms, as desired.
Combinations of shapes can be used to achieve better placement. Where a
plurality of mechanisms is used, the mechanisms can be formed in an organized
pattern (e.g., rows, circles, etc.) or a disorganized (random) pattern is a
cone shaped portion provided on the undersurface of the implant. Further, where
more than one attachment mechanism is used the orientation relative to one
another can be parallel or non-parallel.
[0217]
In one example a
cone is positioned on the undersurface of the device such that it is placed at
the bottom of, for example, the concave part of the tibial cartilage. The cone
can, like the sphere, also be separated from the undersurface of the implant
by, for example, a cylindrical element. Other geometries suitable for
attachment will be apparent to those of skill in the art.
[0218]
In another example,
one or more cylindrical, or substantially cylindrical, pins are provided on a
surface of an implant. The pins are oriented such that each pin is parallel to
at least one other pin.
[0219]
Yet another example
for a semi-fixed attachment mechanism is a magnet which is placed underneath
the subchondral bone layer, for example in the tibia. Another magnet or
magnetic material is embedded into or attached to the undersurface of the
device, which is then held in place by the first magnet. As will be appreciated
by a person of skill in the art, a plurality of magnets associated with each
surface can be used. Further, a combination of magnets can be used such that
each surface has one or more magnets having a first pole and one or more
magnets having a second pole that engage with a magnet with an opposite pole
magnet on, or associated with, the opposing surface. Such an arrangement might
be useful where there is a desire to prevent rotation of the device within the
joint while ensuring communication between the two surfaces.
[0220]
Yet another example
for such attachment mechanisms is a screw or anchor that can be inserted into
the subchondral bone of the tibia at the bottom of the concave portion of the
tibial cartilage. The device can be fixed to the screw or anchor or can have a
semi-fixed design, for example by incorporating a slot which slides over the
screw or anchor.
[0221]
The implant height
can be adjusted to correct articular malalignment or axis deviation(s). For
example, in a knee joint, the articular height can be adjusted to correct for
varus or valgus deformity. Such correction can be determined using measurements
of the axis or axes of the joint as well as neighboring joints. For example, a
CT or MRI scan or a weight-bearing radiograph of the extremity can be used for
this purpose.
[0222]
Implant thickness
can also be selected or adjusted to correct the presence of ligamentous laxity.
In a knee joint, for example, a slightly thicker implant can be selected to
account for laxity or tear of one or more cruciate or collateral ligaments. The
increase in implant thickness can be uniform or non-uniform, e.g. predominantly
at the peripheral margin. The surgeon can use one or more trial prosthesis or
actual implants intraoperatively to test which implant thickness yields the
most preferred result with regard to articular and implant laxity.
[0223]
V. Implantation
[0224]
The devices
described herein are typically implanted into the area of a joint defect.
Implantation can be performed with the cartilage replacement or regenerating
material still attached to the base material or removed from the base material.
Any suitable methods and devices can be used for implantation, for example,
devices as described in U.S. Pat. No. 6,375,658 to Hangody, et al. issued Apr.
23, 2002; U.S. Pat. No. 6,358,253 to Torrie, et al. issued Mar. 19, 2002; U.S.
Pat. No. 6,328,765 to Hardwick, et al., issued Dec. 11, 2001; and International
Publication WO 01/19254 to Cummings, et al., published Mar. 22, 2001.
[0225]
The implants can be
inserted using arthroscopic assistance. The device does not require the 15 to
30 cm incision utilized in certain unicompartmental and total knee
arthroplasties. The procedure is performed under regional anesthesia, typically
epidural anesthesia. A tourniquet can be applied to a more proximal portion of
the extremity. The region of the body containing the joint to be repaired is
prepped and draped using a sterile technique. In the case of the knee, for
example, a stylette is used to create two small 2 mm ports at the anteromedial
and the anterolateral aspect of the joint using classical arthroscopic
technique. The arthroscope is inserted via the lateral port. The arthroscopic
instruments are inserted via the medial port. A cartilage defect can be
visualized using the arthroscope. A cartilage defect locator device can be
placed inside the diseased cartilage. The probe can have a U-shape, with the
first arm touching the center of the area of diseased cartilage inside the
joint and the second arm of the U remaining outside the joint. The second arm
of the U indicates the position of the cartilage relative to the skin. The
surgeon marks the position of the cartilage defect on the skin. A 3 cm incision
is created over the defect. Tissue retractors are inserted and the defect is
visualized.
[0226]
The implant is then
inserted into the joint. Anterior and posterior positions of the implant can be
color-coded. For example, the anterior peg can be marked with a red color and a
small letter “A”, while the posterior peg can be green color and a marked with
a small letter “P”. Similarly, the medial aspect of the implant can be
color-coded yellow and marked with a small letter “M” while the lateral aspect
of the implant can be marked with a small letter “L”.
[0227]
Areas of cartilage
can be imaged as described herein to detect areas of cartilage loss and/or
diseased cartilage. The margins and shape of the cartilage and subchondral bone
adjacent to the diseased areas can be determined. The thickness of the
cartilage can be determined. The shape of the menisci or other articular
structures can be determined. The size and shape of the device is determined
based on one or more of the above measurements. In particular, the repair
system is either selected (based on best fit) from a catalogue of existing,
pre-made implants with a range of different sizes and curvatures or
custom-designed or patient specific using CAD/CAM technology. The custom
designed implant can be generated using one or more patient dependent
parameters. The patient dependent parameters can be obtained using one or more
measurements of the patient's joint to be repaired. Further, the library of
existing shapes can be on the order of about 30 sizes. As will be appreciated
by those of skill in the art, the library can contain more than 30 shapes or
less than 30 shapes, if desired, without departing from the scope of the
invention.
[0228]
More specifically,
to implant a device within the hip joint, the surgeon would make a small
incision as described above. Tissue retractors as well as other surgical
instruments as are commonly used for hip surgery can be used in order to expose
the hip joint. The capsule can be opened subsequently. A second surgeon can pull
on the femur or tibia in order to open up the space between the femoral head
and the acetabulum. The primary surgeon performing the procedure can then
insert the arthroplasty device into the joint. If necessary, the surgeon can
cut the ligamentum capitis femoris and debride portions of the articular
surface, for example in order to remove torn labral tissue or cartilage flaps.
The surgeon also has the option to remove the fat located in the area of the
pulvinar.
[0229]
Alternatively, where
the arthroplasty system is composed of a self-expandable material, e.g.
Nitinol, the surgeon can obtain entry to the hip via a standard or a modified
arthroscopic approach. The implant can then be delivered via the same or a
second portal or, alternatively, via a small incision. Once inside the joint,
the implant can expand and take its final shape. In order to facilitate
placement of the expandable implant, a guide or mold can be used. The guide or
mold can be adapted to the 3D contour of the femoral or acetabular articular
surface and can be placed in the intended position for the implant. The implant
can then be advanced along the guide or, for example, within a hollow chamber
inside the guide or mold. Once the implant has reached its intended position,
the guide or mold can be removed with the implant remaining in place.
[0230]
VI. Device Molds
[0231]
In another
embodiment of the invention, a container or well can be formed to the selected
specifications, for example to match the material needed for a particular
subject or to create a stock of repair and/or materials in a variety of sizes.
The size and shape of the container can be designed using the thickness and
curvature information obtained from the joint and from the cartilage defect.
More specifically, the inside of the container can be shaped to follow any
selected measurements, for example as obtained from the cartilage defect(s) of
a particular subject. The container (mold) can be filled with a replacement
material to form the device that will be implanted.
[0232]
Molds can be
generated using any suitable technique, for example computer devices and
automation, e.g. computer assisted design (CAD) and, for example, computer
assisted modeling (CAM). Because the resulting material generally follows the
contour of the inside of the container it can better fit the defect itself and
facilitate integration.
[0233]
VII. Implantation
Guides and Surgical Tools
[0234]
The molds described
above can also be used to design surgical implantation guides and tools having
at least one outer surface that matches or nearly matches the contour of the
underlying articular surface (bone and/or cartilage). In certain embodiments, two
or more outer surfaces match the corresponding articular surfaces. The tool as
a whole can be round, circular, oval, ellipsoid, curved or irregular in shape.
The shape can be selected or adjusted to match or enclose an area of diseased
cartilage or an area slightly larger than the area of diseased cartilage.
Alternatively, the tool can be designed to be larger than the area of diseased
cartilage. The tool can be designed to include the majority of or the entire
articular surface. Two or more tools can be combined, for example for two or
more articular surfaces.
[0235]
One or more
electronic images can be obtained providing object coordinates that define the
articular and/or bone surface and shape. The biomechanical axes of the joint
can also be defined, for example using an imaging test such as a CT or MRI scan
or a standing, weight-bearing radiograph. For example, if surgery is
contemplated for a knee joint, a CT scan or a spiral CT scan can be obtained
through the knee joint. The CT scan can be limited to the knee joint region and
the distal femur and proximal tibia. Alternatively, the scan can include images
through the hip joint and, optionally, also the ankle joint. In this manner,
the anatomic axes can be defined and the preferred planes for surgical placement
of a knee implant can be selected. The scan can be contiguous.
[0236]
Alternatively,
selected scan planes can be obtained through the hip and ankle region in order
to define the anatomic axes. The CT scan can be combined with intra-articular
contrast administration in order to visualize the articular cartilage. In
another example, a non-contrast CT scan can be used. If no contrast is used,
the residual cartilage thickness can be estimated, for example, using a
reference database of age, sex, race, height and weight matched individuals. In
advanced arthritis, a reduction in normal cartilage thickness can be assumed.
For example, in a knee joint, cartilage thickness can be assumed to be zero or
near zero in the weight-bearing region in patients with advanced arthritis,
while in the posterior non-weight-bearing regions a value of 2 mm or less can
be selected. These estimated cartilage thickness can then be added to the
curvature of the subchondral bone to provide an estimate of the shape of the
articular surface. If an MRI is used, a high resolution scan can be obtained
through the knee in which the surgeon is contemplating the surgery. This scan
is advantageous for defining the articular geometry. The high resolution scan
can be supplemented with a scan using lower resolution through adjacent joints
and bones in order to define the anatomic axes.
[0237]
If a total knee
arthroplasty is contemplated, the high resolution scan can be acquired in the
knee joint, while lower resolution scans can be acquired in the hip joint and,
optionally, the ankle joint. Such lower resolution scans can be obtained with
the body coil or a torso phased array coil.
[0238]
Imaging tests can
also be combined. For example, a knee MRI scan can be used to define the 3D
articular geometry of the knee joint including subchondral bone and cartilage.
The knee MRI scan can be combined with a standing, weight-bearing x-ray of the
extremity that describes the anatomic axes. In this manner, object coordinates
and anatomic axes can be derived that can be used to define the preferred
planes for surgical intervention.
[0239]
Object coordinates
can be utilized to either shape the device to adapt to the patient's anatomy,
e.g. using a CAD/CAM technique or, alternatively, to select a pre-made device
that has a good fit with a patient's articular anatomy. As noted above, the
tool can have a surface and shape that can match all or portions of the
articular or bone surface and shape, e.g. similar to a “mirror image” of the
device to be implanted. The tool can include apertures, slots and/or holes to
accommodate surgical instruments such as drills and saws and the like. The tool
can be used for partial articular replacement as well as total joint
replacement. For example, in total knee arthroplasty, the tool can be used for
accurate placement of the cut planes needed for implant insertion. In this
manner, a more reproducible implant position can be achieved with the potential
to improve clinical outcomes and long-term implant survival.
[0240]
The tool can have
one, two or more components. One part of the tool can be made of metal while
other can be made of plastic. For example, the surface that is touching the
articular surface during the surgery can be made of plastic. In this manner, it
is easy and cheap to manufacture, for example using rapid prototyping
techniques. The plastic component can be made individually for each patient or
pre-selected from a range of existing size. The portion(s) of the plastic
component that points away from the articular surface can have the same surface
geometry, e.g. block-like, in all patients. In this manner, a pre-fabricated
metal component can be applied to the plastic component. The metal component
can include the surgical guides, e.g. openings for saws or drills. The plastic
component will typically have openings through which the surgical instruments
can be advanced to the bone or cartilage without having to damage the plastic.
[0241]
The plastic
component determines the position of the metal component and surgical guides in
relation to the articular surface. Spacers can be introduced between both
components, for example in order to adjust the depth of bone cuts. Thus, in a
knee joint, the surgeon can test for flexion and extension gap and, using the
spacers, adjust the gaps and select the most appropriate cut planes. Moreover,
if two or more components are used, rotational adjustment can be allowed
between the components. In this manner, the surgeon can, for example, balance
the medial and lateral compartments in a knee joint. After any optional
rotational adjustments have been made, the components can be fixed relative to
each other or relative to the bone or cartilage before the surgeon places any
cuts or makes any other manipulations.
[0242]
The component(s) and
tools can be designed to be compatible with existing surgical instrument sets
used for arthroplasty, e.g. total knee arthroplasty. Notably, the tool(s) can
help reduce the number of surgical instruments used for arthroplasty. Finally,
this embodiment can help improve postoperative alignment of the implant(s)
relative to the desired location or anatomic axes thereby decreasing prosthetic
loosening, implant wear, stresses on bone and thereby improving long-term
outcomes.
[0243]
Typically, a
position is chosen that can result in an anatomically desirable cut plane or
drill hole orientation for subsequent placement of an implant. Moreover, the
guide device can be designed so that the depth of the drill or saw can be
controlled, e.g., the drill or saw cannot go any deeper into the tissue than
defined by the thickness of the device, and the size of the hole in block can
be designed to essentially match the size of the implant. Information about
other joints or axis and alignment information of a joint or extremity can be
included when selecting the position of these slots or holes. The guides can be
prepared for any of the implants of the invention.
[0244]
Turning now to
specific examples of implantation guides shown in FIGS. 28 and 17, these
examples are provided for illustration purposes. FIG. 28 illustrates a plan
view of an implantation guide 1100 suitable for use with the
implant shown in FIG. 8L. A joint conforming body is provided 1110. The joint
conforming body is configured to have at least one exterior surface configuration
that matches an exterior surface configuration of the implant 100 to
be used. A handle 1112 is provided to enable the user to place
the guide in the joint where the implant 100 will be placed.
Additionally, an anchor guide 1114 is provided. In this instance
the anchor guide 1114 is in an opening within
the body 1110 in the shape of a cross. As will be appreciated by
those of skill in the art, the anchor guide 1114 can assume a
variety of shapes, as appropriate, to enable the guide to perform its intended
function. In this instance, the cross-shape enables the user to identify the
articular surface of the joint where the anchor 112 (shown in FIG.
3L) is positioned on the joint. Once the guide 1100 is placed on
the target articular surface, the anchor guide 1114 can be used
to either: mark the location where the anchor can access the joint; confirm the
location where the anchor can access the joint; prepare the articular surface
at the location where the anchor can be located; or a combination thereof.
[0245]
Turning now to
the guide 1200 shown in FIG. 25A-B, plan views of a guide
suitable for use with the implant shown in FIG. 9A-C are shown. A body is
provided 1210. The body is configured to have at least one exterior surface
that matches, or nearly matches, an exterior surface configuration of
an implant 150 to be implanted. A handle 1212 is
provided to enable the user to place the guide on a joint surface where
the implant 150 can be placed. Additionally, one or more anchor
guides 1214 are provided. In this instance the anchor guides 1214 (1214′, 1214″, 1214′″)
are circular, or substantially circular, openings within the body 1210 that
are large enough in diameter to accept the drill bits for drilling the holes
inside the bone in which the pins of the anchors 156 of
the implant 150 will be placed. As will be appreciated by those
of skill in the art, the anchor guide 1214 can assume a variety
of shapes, as appropriate, to enable the guide to perform its intended
function. Additional guides 1216 can be provided. The additional
guides can perform the same function as the primary guides 1214 or
can perform a secondary function. In this instance, the anchor guides 1214 can
be used to identify the articular surface of the joint where the anchors 156 (shown
in FIG. 9B-C) can be positioned on the joint. Once the guide 1200 is
placed on the target articular surface, the anchor guide 1214 can
be used to either; mark the location where the anchor can be access the joint;
confirm the location where the anchor can access the joint; prepare the
articular surface at the location where the anchor can be located; or a
combination thereof. Additionally, guides 1216 can be used to mark
the location where the anchor can access the joint; confirm the location where
the anchor can access the joint; prepare the articular surface at the location
where the anchor can be located; or a combination thereof.
[0246]
In another
embodiment, a frame can be applied to the bone or the cartilage in areas other
than the diseased bone or cartilage. The frame can include holders and guides
for surgical instruments. The frame can be attached to one or preferably more
previously defined anatomic reference points. Alternatively, the position of
the frame can be cross-registered relative to one, preferably more anatomic
landmarks, using an imaging test, for example one or more fluoroscopic images
acquired intraoperatively. One or more electronic images can be obtained
providing object coordinates that define the articular and/or bone surface and shape.
These objects coordinates can be entered or transferred into the device, for
example manually or electronic, ally, and the information can be used to move
one or more of the holders or guides for surgical instruments. Typically, a
position is chosen that can result in a surgically or anatomically desirable
cut plane or drill hole orientation for subsequent placement of an or other
implant including hemi-, unicompartmental or total arthroplasty. Information
about other joints or axis and alignment information of a joint or extremity
can be included when selecting the position of these slots or holes.
[0247]
Because of its
anatomic alignment with the chosen underlying articular surface, the preferred
location and orientation of saw guides, drill holes or guides for reaming
devices can be created in the appropriate tool. Intraoperatively, the surgical
assistance tool is applied to the articular surface(s) with which it achieves
the near or perfect anatomic fit. The surgeon can then introduce a saw (or
other tool) through the guide(s) and prepare the joint (cartilage or bone) for
the procedure. By cutting the cartilage and/or bone along anatomically defined
planes, a more reproducible placement can be achieved which ultimately leads to
improved postoperative results by optimizing biomechanical stresses.
[0248]
The anatomically
correct tools described herein can be constructed by a number of methods and
can be made of any material, preferably a translucent material such as plastic,
Lucite, silastic, SLA or the like, and typically is a block-like shape prior to
molding. Furthermore, re-useable tools (e.g., molds) can be also be created and
employed. Non-limiting examples of re-useable materials include putties and
other deformable materials (e.g., an array of adjustable closely spaced pins
that can be configured to match the topography of a joint surface). In these embodiments,
the mold can be created directly from the joint during surgery or,
alternatively, created from an image of the joint, for example, using one or
more computer programs to determine object coordinates defining the surface
contour of the joint and transferring (e.g., dialing-in) these co-ordinates to
the tool. Subsequently, the tool can be aligned accurately over the joint and,
accordingly, the drill and implant can be more accurately placed in and over
the articular surface.
[0249]
In both single-use
and re-useable embodiments, the tool can be designed so that the depth of the
block controls the depth of the drill or saw, i.e., the drill or saw cannot go
any deeper into the tissue than the depth of block, and the size of the hole in
block can be designed to essentially match aspects of the size of the implant.
The tool can be used for general prosthesis implantation, including, but not
limited to, the articular repair implants described herein and for reaming the
marrow in the case of a hemi-, unicompartmental or total arthroplasty or other
articular systems including biological repair.
[0250]
These surgical tools
can also be used to remove an area of diseased cartilage or an area slightly
larger than the diseased cartilage.
[0251]
Identification and preparation
of the implant site and insertion of the implant can be supported by an
image-guided surgery system (surgical navigation system). In such a system, the
position or orientation of a surgical instrument with respect to the patient's
anatomy can be tracked in real-time in one or more 2D or 3D images. These 2D or
3D images can be calculated from images that were acquired preoperatively, such
as MR or CT images. The position and orientation of the surgical instrument is
determined from markers attached to the instrument. These markers can be
located by a detector using, for example, optical, acoustical or
electromagnetic signals. Surgical navigation systems can also be used without
image guidance, for example, by identifying anatomic axes with use of motion
studies of an extremity.
[0252]
In still other
embodiments, the surgical tools described herein can include one or more
materials that harden to form a mold of the articular surface. A wide-variety
of materials that harden in situ have been described including polymers that
can be triggered to undergo a phase change, for example polymers that are
liquid or semi-liquid and harden to solids or gels upon exposure to air,
application of ultraviolet light, visible light, exposure to blood, water or
other ionic changes. (See, also, U.S. Pat. No. 6,443,988 and documents cited
therein). Non-limiting examples of suitable curable and hardening materials
include polyurethane materials (e.g., U.S. Pat. No. 6,443,988 to Felt, et al.,
issued Sep. 3, 2002; U.S. Pat. No. 5,288,797 to Khalil, et al., issued Feb. 22,
1994; U.S. Pat. No. 4,098,626 to Graham, et al., issued Jul. 4, 1978, and U.S.
Pat. No. 4,594,380 to Chapin, et al., issued Jun. 10, 1986; and Lu et al.
(2000) BioMaterials 21(15):1595-1605 describing porous
poly(L-lactide acid foams); hydrophilic polymers as disclosed, for example, in
U.S. Pat. No. 5,162,430 to Rhee, et al., issued Nov. 10, 1992 hydrogel
materials such as those described in Wake et al. (1995) Cell
Transplantation 4(3):275-279, Wiese et al. (2001) J. Biomedical
Materials Research 54(2):179-188 and Marler et al. (2000) Plastic
Reconstruct. Surgery 105(6):2049-2058; hyaluronic acid materials (e.g.,
Duranti et al. (1998) Dermatologic Surgery 24(12):1317-1325);
expanding beads such as chitin beads (e.g., Yusof et al. (2001) J.
Biomedical Materials Research 54(1):59-68); and/or materials used in
dental applications (See, e.g., Brauer and Antonucci, “Dental Applications” pp.
257-258 in “Concise Encyclopedia of Polymer Science and Engineering” and U.S.
Pat. No. 4,368,040 to Weissman, issued Jan. 11, 1983). Any biocompatible
material that is sufficiently flowable to permit it to be delivered to the
joint and there undergo complete cure in situ under physiologically acceptable
conditions can be used. The material can also be biodegradable.
[0253]
The curable
materials can be used in conjunction with a surgical tool as described herein.
For example, the surgical tool can include one or more apertures therein
adapted to receive injections and the curable materials can be injected through
the apertures. Prior to solidifying in situ the materials can conform to the
articular surface facing the surgical tool and, accordingly, can form an
impression of the surface upon hardening thereby recreating a normal or near
normal articular surface. In addition, curable materials or surgical tools can
also be used in conjunction with any of the imaging tests and analysis
described herein, for example by molding these materials or surgical tools
based on an image of a joint.
[0254]
Turning now to FIG.
27 A-D, the steps of the method of implanting the devices taught in this
invention are shown. First, the user makes an incision to access the target
joint 2610. Thereafter the joint surface is prepared using
the implant guide 2620. Preparation of the joint surface can include,
for example, identifying where the implant can reside in the joint, marking
where the implant can attach, and/or preparing the articular surface to receive
the implant. This preparation process can be repeated as necessary. As those of
skill in the art will understand, in preparing the joint surface, the user can
first identify where the implant will reside and then prepare the surface by
marking the articular surface or removing bone or cartilage. Once the surface
of the joint has been prepared, the implant is installed 2640. Installing
the implant can be by either placing the implant on the surface or by adhering
the implant to the surface using the techniques described herein. After the
implant has been installed within the joint, the wound is closed 2650.
[0255]
Turning now to the
steps shown in FIG. 27 B, the user makes an incision to access the target
joint 2610. Thereafter a frame is attached to the joint 2660. Although
not shown in this flow chart, the steps of preparing the joint shown in FIG.
27A can be performed. The implant is then installed 2665 onto
the frame. After the implant has been installed within the joint, the wound is
closed 2650.
[0256]
Turning now to the
steps shown in FIG. 27 C, the user makes an incision to access the target
joint 2610. Thereafter diseased cartilage is removed from the joint 2670.
Although not shown in this flow chart, the additional steps of preparing the
joint shown in FIG. 27A can also be performed without departing from the
scope of the invention. The implant is then installed 2675. After the
implant has been installed within the joint, the wound is closed 2650.
[0257]
Turning now to the
steps shown in FIG. 27 D, the user makes an incision to access the target
joint 2610. Although not shown in this flow chart, the additional steps of
preparing the joint shown in FIG. 27A can also be performed without
departing from the scope of the invention. Thereafter the implant is
inserted 2680. The position of the implant is then optionally
adjusted 2682. After the implant has been inserted and positioned, the
profile of the implant is adjusted 2684. After the implant has been
installed within the joint and adjusted, the wound is closed 2650. The
implant height or profile selected can be chosen to alter the load bearing
ability relative to the joint. Additionally the implant height can be adjusted
to account for anatomic malalignment of bones or articular structures.
[0258]
VII. Kits
[0259]
Also described
herein are kits comprising one or more of the methods, systems and/or
compositions described herein. In particular, a kit can include one or more of
the following: instructions (methods) of obtaining electronic images; systems
or instructions for evaluating electronic images; one or more computer means
capable of analyzing or processing the electronic images; and/or one or more
surgical tools for implanting an implant. The kits can include other materials,
for example, instructions, reagents, containers and/or imaging aids (e.g.,
films, holders, digitizers, etc.).
[0260]
The following
examples are included to more fully illustrate the present invention.
Additionally, these examples provide preferred embodiments of the invention and
are not meant to limit the scope thereof.
[0261]
The foregoing
description of embodiments of the present invention has been provided for the
purposes of illustration and description. It is not intended to be exhaustive
or to limit the invention to the precise forms disclosed. Many modifications
and variations will be apparent to the practitioner skilled in the art. The
embodiments were chosen and described in order to best explain the principles
of the invention and its practical application, thereby enabling others skilled
in the art to understand the invention and the various embodiments and with
various modifications that are suited to the particular use contemplated. It is
intended that the scope of the invention be defined by the following claims and
its equivalence.
What is claimed:
1. An implant
suitable for a knee joint having a superior surface and an inferior surface
wherein the superior surface opposes at least a portion of a femur and the
inferior surface opposes at least a portion of a tibial surface and further
wherein at least one of the superior or inferior surfaces has a
three-dimensional shape that substantially matches the shape of one of the
femur and tibia surfaces.
2. The implant
of claim 1 wherein the superior surface and the inferior surface have
a three dimensional shape that substantially matches the shape of at least one
of the articular surface that the superior surface of the implant abuts and the
inferior surface of the implant abuts.
3. The implant
of claim 1 wherein the implant has a thickness of a cartilage defect
in a patient.
4. The implant
of claim 1 wherein the implant has a thickness of 85% of a cartilage
defect in a patient.
5. The implant
of claim 1 wherein the implant has a thickness of between 65%-100% of
a cartilage defect of a patient.
6. The implant of claim
1 wherein the implant has a thickness of a cartilage defect plus a
predefined offset value.
7. The implant
of claim 6, wherein said offset value can be selected to adjust for axis
malalignment.
8. The implant
of claim 1 wherein the implant is constructed of a material
comprising metal or metal alloy.
9. The implant
of claim 1 wherein the material comprises one or more biologically
active materials.
10. The implant
of claim 6 wherein the implant is coated with a biologically active
material.
11. The implant
of claim 1 wherein the implant is comprised of a metal or metal alloy
and a polymer.
12. The implant
of claim 1 further having a structure for attachment on at least one
of the superior surface and the inferior surface selected from the group consisting
of: ridges, pegs, pins, cross-members, teeth and protrusions.
13. The implant
of claim 12 further having a plurality of structures for attachment.
14. The implant
of claim 13 wherein the relative orientation of the structures for
attachment are selected from the group consisting of: symmetrical,
asymmetrical, rows, circles, triangles, and random.
15. The implant
of claim 1 further having a peripheral structure selected from the
group consisting of ridges and lips.
16. The implant
of claim 15 wherein the peripheral structure extends along an entire
perimeter of the implant.
17. The implant
of claim 15 wherein the peripheral structure extends along a portion
of a perimeter of the implant.
18. The implant
of claim 1 wherein each of the superior surface and inferior surface
have a slope relative to a longitudinal axis through at least a portion of the
implant and further wherein the slope of the superior surface relative to the
slope of the inferior surface is selected from the group consisting of:
positive, negative, and null.
19. The implant
of claim 1 wherein the implant approximates the shape of one of the
first and second articular surface.
20. The implant
of claim 19 wherein the implant is selected from a library of
implants.
21. The implant
of claim 1 wherein the implant changes configuration after insertion
into a joint.
22. The implant
of claim 1 wherein the implant further comprises a first component
and a second component.
23. The implant
of claim 22 wherein the first component and second component are one
of: integrally formed, indivisibly formed, interconnectedly formed, and
interdependently formed.
24. The implant
of claim 22 wherein the first component engages the joint in at least
one of fixedly, slideably, rotatably.
25. The implant
of claim 22 wherein the second component engages the joint in at
least one of fixedly, slidably, and rotatably.
26. The implant of
claims 22, 23, 24 and 25 wherein the first
component engages the second component.
27. The implant of
claims 22, 23, 24 and 25 wherein the first
component fits within the second component.
28. The implant of
claims 22, 23, 24 and 25 wherein the first
component slideably engages the second component.
29. The implant of
claims 22, 23, 24 and 25 wherein the first
component rotatably engages the second component.
30. The implant of
claims 1, 22, 23, 24 and 25 wherein a
portion of the implant has a magnet.
31. The implant
of claim 1 wherein the implant has a plurality of components.
32. The implant
of claim 31 wherein a first component of the plurality of components
engages the joint in at least one of fixedly, slideably, and rotatably.
33. The implant
of claim 31 wherein a second component of the plurality of components
engages the joint in at least one of fixedly, slidably, and rotatably.
34. The implant of
claims 31, 32 and 33 wherein the first component of
the plurality of components engages the second component of the plurality of
components.
35. The implant of
claims 31, 32 and 33 wherein the first component of
the plurality of components fits within the second component of the plurality
of components.
36. The implant of
claims 31, 32 and 33 wherein the first component of
the plurality of components slideably engages the second component of the
plurality of components.
37. The implant of
claims 31, 32 and 33 wherein the first component of
the plurality of components rotatably engages the second component of the
plurality of components.
38. The implant
of claim 1 wherein the implant has a shape formed along a perimeter
selected from the group consisting of: circular, elliptical, ovoid, kidney
shaped, square, rectangular, substantially circular, substantially elliptical,
substantially ovoid, substantially kidney shaped, substantially square, and
substantially rectangular.
39. The implant
of claim 1 wherein the implant has a cross-sectional shape of at
least one of an inferior surface and a superior surface selected from the group
consisting of spherical, hemispherical, aspherical, convex, concave,
substantially convex, and substantially concave.
40. The implant
of claim 1 wherein the implant is a cartilage defect conforming
implant.
41. The implant
of claim 1 wherein the implant is a cartilage projected implant.
42. The implant
of claim 1 wherein the implant is a subchondral bone conforming
implant.
43. The implant
of claim 1 wherein the implant is surgically implanted via an
incision of 10 cm or less.
44. The implant
of claim 1 wherein the implant is surgically implanted via an
incision of 6 cm or less.
45. The implant
of claim 1 wherein the implant is surgically implanted via an
incision of 4 cm or less.
46. The implant
of claim 1 wherein the range of motion of the joint is restored to
between 80-99.9% of normal joint motion.
47. The implant
of claim 1 wherein the range of motion of the joint is restored to
between 90-99.9% of normal joint motion.
48. The implant
of claim 1 wherein the range of motion of the joint is restored to
between 95-99.9% of normal joint motion.
49. The implant
of claim 1 wherein the range of motion of the joint is restored to
between 98-99.9% of normal joint motion.
50. The implant
of claim 1 wherein a t least a portion of a periphery of the implant
is of greater thickness than a central portion of the implant.
51. The implant
of claim 1 wherein a central portion of the implant is of greater
thickness than at least a portion of a periphery.
52. The implant
of claim 1 wherein the margin of the implant is rounded in one or
more locations.
53. The implant
of claim 1 wherein the superior margin extends further medially,
laterally, anteriorly and/or posteriorly than the inferior margin.
54. The implant
of claim 1 wherein the inferior margin extends further medially,
laterally, anteriorly and/or posteriorly than the superior margin.
55. The implant of
claims 1, 22 and 31 having an anterior portion,
posterior portion, lateral portion and medial portion wherein the implant has a
thickness along at least one of the anterior portion, posterior portion,
lateral portion and medial portion of the device that is equal to or greater
than a thickness of at least one of the lateral, medial, anterior and posterior
portion of the implant.
56. The implant of
claims 1, 22 and 31 having an anterior portion,
posterior portion, lateral portion and medial portion wherein the implant has a
thickness along at least one of the anterior portion, posterior portion,
lateral portion and medial portion of the device that is equal to or less than
a thickness of at least one of the lateral, medial, anterior and posterior
portion of the implant.
57. An implant
suitable for a hip joint having a superior surface and an inferior surface
wherein the superior surface engages at least a portion of an acetabulum and
the inferior surface engages a least a portion of a head of a femur and further
wherein at least a portion of at least one of the superior or inferior surfaces
has a three-dimensional shape that substantially matches the shape of one of
the acetabulum and head of the femur surfaces.
58. An implant
suitable for a hip joint having a superior surface and an inferior surface
wherein the superior surface engages at least a portion of a head of the femur
and the inferior surface engages a least a portion of an acetabulum and further
wherein at least a portion of at least one of the superior or inferior surfaces
has a three-dimensional shape that substantially matches the shape of one of the
acetabulum and head of the femur surfaces.
59. An implant
suitable for an ankle joint having a superior surface and an inferior surface
wherein the superior surface engages at least a portion of a distal tibia and
the inferior surface engages at least a portion of a talar dome and further
wherein at least a portion of at least one of the superior or inferior surfaces
has a three-dimensional shape that substantially matches the shape of one of
the distal tibia and talar dome surfaces.
59. An implant suitable
for an ankle joint having a superior surface and an inferior surface wherein
the superior surface engages at least a portion of a talar dome and the
inferior surface engages at least a portion of a distal tibia and further
wherein at least a portion of at least one of the superior or inferior surfaces
has a three-dimensional shape that substantially matches the shape of one of
the distal tibia and talar dome surfaces.
60. An implant
suitable for a toe joint having a proximal surface and a distal surface wherein
the proximal surface engages at least a portion of a metatarsal head and the
distal surface engages at least a portion of a proximal phalanx and further
wherein at least a portion of at least one of the proximal or distal surfaces
has a three-dimensional shape that substantially matches the shape of one of
the metatarsal head and proximal phalanx surfaces.
61. An implant
suitable for a shoulder joint having a first surface and a second surface
wherein the first surface engages at least a portion of a humeral head and the
second surface engages at least a portion of a glenoid fossa and further
wherein at least a portion of at least one of the first or second surfaces has
a three-dimensional shape that substantially matches the shape of one of the humeral
head and glenoid fossa surfaces.
62. An implant
suitable for an elbow joint having a first surface and a second surface wherein
the first surface engages at least a portion of a distal humerus and the second
surface engages at least a portion of an at least one of an ulna and radius and
further wherein at least a portion of at least one of the first or second
surfaces has a three-dimensional shape that substantially matches the shape of
one of the distal humerus, ulna and radius surfaces.
63. An implant
suitable for a wrist joint having a first surface and a second surface wherein
the first surface engages at least a portion of a distal radius and the second
surface engages at least a portion of a distal ulna and further wherein at
least a portion of at least one of the first or second surfaces has a
three-dimensional shape that substantially matches the shape of one of the
distal radius an distal ulna surfaces.
64. An implant
suitable for a finger joint having a first surface and a second surface wherein
the first surface engages at least a portion of a metacarpal head and the
second surface engages at least a portion of a base of a proximal phalanx and
further wherein at least a portion of at least one of the first or second
surfaces has a three-dimensional shape that substantially matches the shape of
one of the metacarpal head and proximal phalanx surfaces.
65. An
interpositional implant suitable for a knee joint having a superior surface and
an inferior surface wherein the superior surface opposes at least a portion of
a femur and the inferior surface opposes at least a portion of a tibial surface
and further wherein at least a portion of at least one of the superior or
inferior surfaces has a three-dimensional shape that substantially matches the
shape of one of the femur and tibia surfaces.
66. An implant
suitable for the joint of a mammal having a first surface and a second surface
wherein the first surface opposes at least a portion of a first articular
surface and the second surface opposes at least a portion of a second articular
surface and further wherein at least a portion of at least one of the first or
second surfaces has a three-dimensional shape that substantially matches the
shape of one of the femur and tibia surfaces.
External links
Lang P, Steines D, Bouadi H, Miller D. Minimally
invasive joint implant with 3-dimensional geometry matching the articular
surfaces. US20040133276A1 October 7, 2003. 2004. patents.google
Publications
of invention
ATE497740 (T1)
AU2003284035 (A1)
CA2501041 (A1)
EP1555962 (A1)
EP1555962 (B1)
HK1072710 (A1)
JP2006501977 (A)
TW200412918 (A)
TWI231755 (B)
US2004133276 (A1)
US2004138754 (A1)
US2011066245
US2014250676 (A1)
US7799077 (B2)
US8709089 (B2)
US9872773 (B2)
WO2004032806 (A1)
WO2004032806 (A9)
2004LangP_MillerD
Authors & Affiliations
Philipp Lang, Lexington,
MA (US)
Daniel Steines, Palo
Alto, CA (US)
Hacene Bouadi, Palo
Alto, CA (US)
David Miller, Palo
Alto, CA (US)
Barry Linder, Danville,
CA (US)
Cecily Snyder, East Falmouth, MA (US)
Keywords
ligamentum capitis femoris, ligamentum teres, ligament of head of femur, endoprosthesis, prosthesis, invention, unipolar, subtotal
NB! Fair practice / use: copied for the purposes of criticism, review, comment, research and private study in accordance with Copyright Laws of the US: 17 U.S.C. §107; Copyright Law of the EU: Dir. 2001/29/EC, art.5/3a,d; Copyright Law of the RU: ГК РФ ст.1274/1.1-2,7






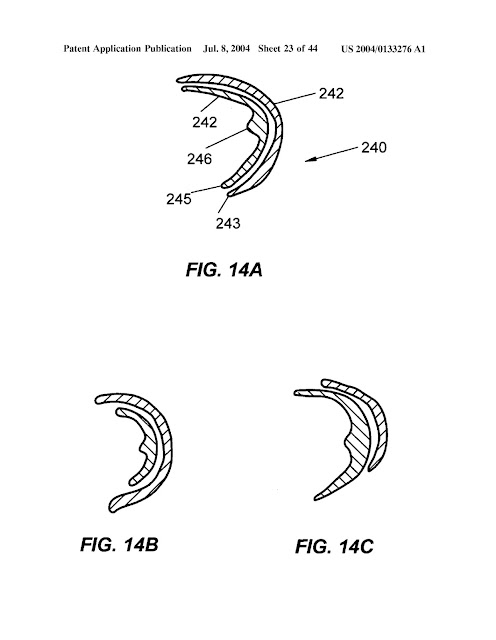







Comments
Post a Comment