Ligamentum Teres and its Analog in the Hip Endoprosthesis–Necessary or
Superfluous? A Systematic Review
S.V. Arkhipov, D.V. Skvortsov
SUMMARY
Background. Dislocation of hip
endoprosthesis remains a common and serious complication of arthroplastic
interventions. One of the ways to prevent endoprosthesis dislocation is to
integrate a ligamentum teres analog into its design.
Purpose. Reviewing
international experience in the design, development and insertion of hip endoprosthesis
with the native ligamentum teres or its analog.
Material
and methods. A systematic patent and non-patent search and analysis of publications
on hip endoprostheses with native ligamentum teres or its artificial analog.
The search was done on relevant online platforms and in available libraries.
Results. To date, there are
20 identified patents on endoprosthesis designs with the native ligamentum
teres or its analog. Ligamentum teres analogs are proposed to be created using
auto-, allo- or xenografts, synthetic materials and metals. We have found two
subtotal endoprosthesis with ligamentum teres analogs that are used in clinical
practice. The long-term outcomes of such surgeries are not known. There are no
commercially available endoprostheses with ligamentum teres analogs.
Conclusions. A ligamentum teres
analog integrated into a hip endoprosthesis can help prevent dislocation in the
post-operative period. Further theoretical, experimental, biomechanical and
clinical studies are needed to develop such endoprostheses for a wider use.
Level of the evidence: 1a
KEY
WORDS: hip joint, ligamentum capitis femoris, ligamentum teres,
endoprosthesis, complication, dislocation of a hip joint prosthesis
INTRODUCTION
Hip
arthroplasty has been widely used since the mid-1940s – early 1950s [1-5]. Soon
after the start of a wide use of both cups and the more familiar
endoprostheses, however, surgeons began to report cases of their dislocation
and subluxation [6-13]. Dislocation after hip replacement is a serious and
devastating complication of such surgical interventions [14, 15]. According to
literature data, the incidence of dislocation reached 15% in the 1970s-1980s
16. These days, dislocation after primary arthroplasty occurs in 0.2-10% of the
cases, more often for prostheses implanted after femoral neck fracture [17-22].
The incidence of dislocation after revision surgeries can reach 25-31% [20, 22,
23]. The risk of dislocation increases with time, regardless the type of
surgery and in spite of adequate restoration of soft tissues [24, 25]. At the
same time, no relationship has been noted between the dislocation rate, on the
one hand, and the patients’ sex, age, diagnosis, and the type of
endoprosthesis, on the other [26]. Surgery for dislocation increases the
treatment costs and health risks for the patient [15]. This problem has
important clinical and social implications and has no clear solution so far.
A
technical solution that can apparently prevent dislocation of endoprosthesis
head is to make a non-detachable ball-and-socket joint. In 1952, H. G. Van
Steenbrugghe was among the first to propose a total endoprosthesis of such a
design, with the femoral component fixed by neck [27]. In order to – among
other things – reduce the risk of displacement for the acetabulum component, J.
Charnley has begun implanting prostheses with a smaller head since 1960, but he
still reports a dislocation rate of 1.5% in the early postoperative period [28,
29]. Dislocation is currently prevented by using non-detachable systems. While
solving the main problem, however, their use is associated with excessive wear
of the friction pair, degradation of the bone-cement and cement-metal bonds,
leading to aseptic loosening and a catastrophic failure of the acetabulum
component [14]. Even at the early stages of implant arthroplasty, one of the
ways for solving the problem of hip implant dislocation was by completing the
implant design with an analog of the ligamentum teres (LT). The first hip endoprosthesis
of this type was proposed by Leon L. Pellet in 1954 [30].
The use
of native LT or its analog as a binding element in the endoprosthesis design
has not been fully developed yet. The purpose of this paper was to review
relevant international experience, from the concept formation to the
development and practical use of hip prostheses with native LT or its analogs,
and to understand the prospects for their use and improvement. Currently, there
are no systematic reviews on design of hip endoprostheses with the LT or its
analogs, registered as projects in PROSPERO and COCHRANE.
MATERIAL
AND METHODS
This
systematic review was done in accordance with the PRISMA (Preferred Reporting
Items for Systematic Reviews and Meta-analyses) Guidelines [31]. The Protocol
of Systematic Review of the topic in question was prepared and discussed by the
team of authors. The Protocol are available upon request from the corresponding
author. The basis of our review of international experience in creation and use
of hip endoprostheses with native LT or its analog was the study of patent
literature from the following electronic databases: Google Patents, Mountain
View, CA, USA (https: // patents.google.com); European Patent Organization
(EPO), Rijswijk, Netherlands (https: // worldwide.espacenet.com); World
Intellectual Property Organization (WIPO), New York, NY, USA (https: // www.wipo.int);
Federal Institute of Industrial Property (FIPS), Moscow, Russian Federation
(https://new.fips.ru). Since the literature on the subject is quite scanty, we
did not limit the search by language or publication status. When examining the
identified documents, the authors gave preference to publications in Russian
and English, the languages they knew.
The
initial patent search was performed in the Google Patents electronic database
for the period January 1940—December 2019, inclusive. The eligibility criteria
were as follows: a full-text patent describing the design of any hip prosthesis
with native LT or its structural analog, or an element similar to native LT in
its position and function, located inside the natural hip joint or
endoprosthesis and connecting the implant head or the natural femoral head with
the acetabulum of the pelvis or the acetabulum part of the endoprosthesis. The
search was performed by keywords divided into three groups and connected by the
Boolean operators AND and OR as follows: (“ligament” OR “ligamentum” OR
“ligamentum teres” OR “cord” OR “band” OR “tape”) AND (“hip” OR “hip joint” OR
“acetabulum”) AND (“endoprosthesis” OR “prosthesis” OR “arthroplasty”). The
search was complicated by the lack of a special alphanumeric code for
endoprosthesis with native LT or its analog in the International Patent
Classification (IPC). The closest IPC codes — A61F2002/3233, A61F2002/30688 and
A61F2220/0075 — failed to identify the searched documents definitely. The lack
of common terminology also contributed to search inaccuracies: e.g.,
“endoprosthesis” (or “prosthesis” for short) was also termed as “implant”,
“device”, “socket” and “artificial joint”. The same was observed for the LT,
with its over three dozen synonyms used in the scientific and patent
literature: “ligamentum capitis femoris”, “ligamentum teres femoris”, “ligament
of head of femur”, etc.
During
the preliminary screening, links to patent documents displayed by the search
service were manually evaluated based on the eligibility criteria for the
title, the content of a brief excerpt from the text, and the accompanying
graphic material. Next, each patent document — an abstract or a full text —
that supposedly met the eligibility criteria was formally examined by reading
the text and viewing the illustrative material. If the document met the
eligibility criteria, the related documents were evaluated for compliance with
these criteria: patent and non-patent publications cited therein, patent
documents that cited this document, as well as similar documents from the lists
available on the viewed Internet page (Patent Citations, Non-Patent Citations,
Cited By, Similar Documents). Where the initial search failed to find the
full-text view of a document, the search switched to the electronic platforms
EPO, WIPO and FIPS. These databases were also used to ascertain the dates of
the earliest publications of eligible patent documents. The search for eligible
non-patent scientific publications mentioned in the related documents cited in
patents was done on the Google Scholar electronic platforms
(https://scholar.google.com/), PubMed (https://www.ncbi.nlm.nih.gov/pubmed/),
and in available libraries.
As the
result of our search on these resources, we obtained a cohort of publications
that passed the final screening. Of these, we excluded patent abstracts and
abstracts of scientific reports. Then, from the number full-text publications,
we excluded the descriptions of applications for invention. Next, we identified
and excluded duplicates among the remaining patent descriptions evaluated as
eligible and non-patent publications discussing the installation of the
devices. Thus, we obtained a list of publications for the final analysis.
All the
identified sources of patent information are listed in Table I, in the
order of their earliest priority. The nationality of patent authors was derived
from the two-letter codes of countries, administrative divisions and
intergovernmental organizations (WIPO ST.3). The risk of bias regarding the
applicability and the rational technical essence of the identified hip
prosthesis designs with native LT or its analog was ranked according to the
following criteria: 1) commercial devices used in clinical practice; 2)
small-batch devices used in clinical practice; 3) individually produced devices
used in clinical practice; 4) concepts presented as a text description of the
device design and installation method, with explanatory graphic material, never
used in clinical practice. The maximum risk of bias rating was assigned to
endoprostheses that fell under criterion 4, the minimum, to those under
criterion 1. The risk-of-bias assessment took into account the short-term
outcomes of arthroplasty described in both patent and non-patent documents. All
the identified designs were grouped by the type of endoprosthesis and by the
type of friction pair. Where the materials of friction pairs were either not
specified, or specified too broadly or not quite clearly, the friction pair was
assigned to the most likely type based on the description of the technical
essence of design. Next, we analyzed the options for using native LT or its
analogs in the hip endoprosthesis design, as well as analogs of the external
ligaments of the hip, if available. After finishing the review, we completed
the PRISMA 2009 checklist, which is available in the Appendix (Supporting
information). This systematic review had no external source of funding.
 |
| Table I. Sources of patent information. |
RESULTS
The
systematic search among 9178 patent publications identified 130 patents and
applications for inventions dedicated to hip endoprostheses with native LT or
its analog. Of these, 26 abstracts, 28 applications for inventions and 56
duplicate patent descriptions were excluded at the stages of final screening
and eligibility assessment. Finally, 20 patent documents were included in the
systematic review. From the 55 initially identified non-patent sources
discussing various aspects of hip arthroplasty with an LT analog, including
those describing short-term outcomes of the surgery, we selected two
publications in Russian meeting the eligibility criteria [32, 33].
The
flowchart for selecting information sources (Figure 1) follows the
recommendations of Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA) [31]. Eligible patent documents selected for the
systematic review are presented in Table I.
.jpg) |
| Figure 1. Flowchart of literature search according to PRISMA guidelines (31). |
Of the 20 patents selected for the systematic review, 15 were in English, 3 in Russian, 1 in German and 1 in Chinese.
The
selected patents on hip endoprostheses with native LT or its analogs were
grouped by the earliest priority date and presented in (Figure 2). The
main numbers of patents coming from 90-th years to current time. Therefore, it
is possible to conclude that interest to type of hip endoprostheses with native
LT has been grow up from 90-th and still remain at this level.
.jpg) |
| Figure 2. Patent distribution by earliest priority date. |
We identified 40 authors of patents for invention of hip endoprostheses with native LT or its analog, their nationalities are shown in (Figure 3). The dominated activity - 60% of patents was registered at the US, the second place is 17.5% - Denmark, third (10%) Soviet Union. All the others are less than 3%.
.jpg) |
Figure
3. Nationalities of authors of inventions. |
The risk of bias regarding the applicability and the rational technical essence of identified hip prosthesis designs with native LT or its analogs is ranked in Table II. It is no commercial endoprostheses with LT in this time. A few constructions were applied at clinics; however, results are not clear.
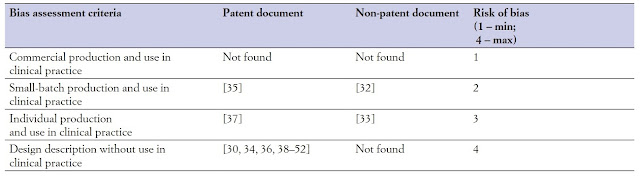 |
| Table II. Risk of bias regarding endoprosthesis applicability. |
The identified designs of endoprostheses with native LT or its analogs were grouped by type and presented in Table III (Figure 4-6).
 |
| Table III. Distribution of endoprostheses by the type of design. |
.jpg) |
Figure
4. Unipolar partial hip endoprosthesis with an artificial LT, which is
indicated by the number 36 (30). |
.jpg) |
Figure
5. Total hip endoprosthesis with an artificial LT, which is indicated by the
sing TL (52). |
Figure 6. Surface replacement arthroplasty with the native LT, which is indicated by the number 56 (43).
The friction pair characteristics and combination of materials are of great importance for the endoprosthesis functioning. The designs of the reviewed hip prostheses with native LT or its analog were grouped by friction pair materials and presented in Table IV.
 |
| Table IV. Distribution of endoprosthesis designs by the type of friction pair. |
DISCUSSION
Most of
the hip endoprosthesis designs with native LT or its analog that passed the
final screening date back to the first decade of the 21st century. That was the
period of extensive introduction of methods for hip arthroscopy and a growing
use of magnetic resonance and computed tomography, and the number of
publications mentioning the LT increased significantly. Another possible
explanation for the fact is that the growing number of hip replacements was
associated with the growing number of implant dislocations. This, again,
stimulated the efforts to improve anti-luxation prostheses.
Based on
the risk of bias regarding the applicability criterion, most of the proposed
devices have the maximum risk rating of 4. We have failed to identify any
commercially available endoprostheses with native LT or its analog, and hence
any devices with the minimum risk rating of 1. Only two subtotal hip
endoprostheses with an LT analog are known to have been used in clinical
practice. The first one — a subtotal endoprosthesis designed by G.E. Dudko —
has been implanted since 1984, and its risk-of-bias can be rated as 2 [35]. The
second one — proposed by V.A. Neverov and V.A. Shilnikov — has been used since
1990 and is assigned a risk-of-bias rating of 3 [37]. The short-term outcomes
of their medical use were assessed as satisfactory [32, 33, 35, 37]. We have
not succeeded in finding any long-term outcomes of these surgical interventions
in the literature.
Scanty
publications about the practical use of hip endoprostheses with an LT analog do
not allow any firm conclusions about their benefits and drawbacks. Of all known
endoprosthesis designs that propose the use of native LT or its analog, 18 have
never been implanted and there are no available reports on their laboratory and
clinical trials.
The
endoprostheses with native LT and its analog included in the review fall under
the existing classification of implants for hip arthroplasty. However, the
specific nature of patent documentation allowed the authors to claim their
proposed technical solution as belonging to several types and, in some cases,
not to specify the type of design at all. We classified 11 of the identified
endoprostheses as total hip endoprostheses (see Table III), 5 as
unipolar partial hip endoprostheses (subtotal endoprosthesis, subtotal hip
prosthesis system), 4 as surface replacement arthroplasty (mould arthroplasty),
2 as partial endoprostheses, and 1 as a spacer prosthesis. We failed to
identify any bipolar partial hip endoprosthesis, revision endoprosthesis or
tumor endoprosthesis (e.g., pelvis tumor-prosthesis) with an LT analog.
Some of
the identified devices (see Table III) for surface replacement
arthroplasty, partial acetabular rim arthroplasty and spacer endoprosthesis are
claimed as intended for retainment of the LT [41, 43, 46, 50]. These
endoprostheses repeat the form of a part of the femoral head articular surface
or the lunate surface of the acetabulum and, generally, have a horseshoe shape.
However, there are some similar devices, of a similar shape (C-shaped, U-shaped,
lunate), whose description has no mentioning of the LT. The technical idea
behind the C-shape is to help preserve the fat pad in the acetabular fossa,
preserve its blood supply, and ensure normal functioning and natural
lubrication of the joint [53, 54].
Most of
the proposed hip endoprostheses with native LT or its analog are supposed to
use a metal head, less often, a polymer one, and quite rarely, a ceramic one
(see Table IV). Some authors propose various embodiments of friction
pair, which do not exclude the use of native cartilage for one of the
components [45, 46, 50] Although some inventions do not specify the type of
friction pair, it can be assumed from the context that their proposed
endoprosthesis has a metal head [30, 36, 38, 39, 49, 51, 52]. Yet other designs
do not clearly state it either, but one can surmise that the endoprosthesis
head or the acetabular liner is made of a polymer material [35, 48, 52]. We
have found design embodiments with a non-detachable articulating system in the form
of an industrially manufactured monobloc [42, 47, 49]. In some endoprostheses,
the articulation system is supposed to be enclosed by an analog of the joint
capsule: a cuff or a cover made of a synthetic material [38]. In rare cases, it
is proposed to use a lubricant in the friction pair [43, 47].
Some
endoprostheses use native LT, others, a modified one, and still others replace
it by a specially designed analog. It is proposed that healthy native LT should
be retained in surface replacement arthroplasty, spacer prosthetics and in
total hip arthroplasty [41, 43, 46, 48, 50]. If the native LT is not healthy,
it can be reinforced with an artificial structure [48]. As noted in the device
descriptions, an LT can be reconstructed using biological tissues (auto-, allo-
or xenografts), polymer materials, and combination thereof [35-37, 39, 42, 44,
45]. There are known proposals to make LT analogs of metal, in the form of
rods, chains or cords [30, 34, 38]. Some designs provide LT analogs with a
protective coating 45, 47, others, with a coating that carries a drug, e.g. an
antibiotic [52]. It has also been proposed to make an external deformable
coating for LT analog and put antibiotics inside [45]. Assuming that an LT
analog can be damaged during its use, various methods for its repair are
discussed [47].
Some
endoprostheses are proposed to be supplemented with artificial analogs of
external ligaments [39, 44, 48]. Here, we should note that there is a method
for creating artificial external ligaments to prevent post-arthroplasty
dislocation. The method was tested in practice and proved useful [55-60]. The
above-said supports the feasibility of supplementing hip prosthesis with native
LT or its analog, analogs of external ligaments, and retaining them whenever possible
during arthroplasty. The most tricky and highly specialized aspect of creating
endoprosthesis with an LT analog is to provide conditions for the element
functioning, proper positioning of its attachment areas, its geometric and
mechanical properties. The lack of fundamental experimental and clinical
studies of these issues makes it impossible to evaluate properly all data
presented in the reviewed sources.
CONCLUSION
Among
patents for inventions, we have identified 20 descriptions of various hip
endoprosthesis systems which include native LT or its analog. At the same time,
there is an apparent new trend in arthroplasty – creation of endoprostheses
that are structurally similar to the natural joint and include the ligamentous
apparatus as their structural component. There is need for further studies of
mechanical impacts on an LT analog and its attachment area, the nature of its
connection with the other parts of the endoprosthesis. These issues are among
the least developed in this field.
Prevention
of post-operative dislocations requires developing new surgical approaches and
methods for placing an endoprosthesis while retaining or adequately
reconstructing the natural ligamentous apparatus. The variety of the already
available designs demonstrates multiple potential solutions to the pressing
problems of arthroplasty, on the one hand, and the scarcity of basic data, on
the other.
Creating
such endoprostheses seems quite realistic. However, it requires extensive
preliminary research and development efforts. These devices can likely make a
line of implants for staged arthroplasty, from simple and small ones, requiring
minimal removal of native tissues, to complex and massive reconstructive and
oncologic endoprostheses. The common distinguishing feature of the
new-generation endoprostheses should be the ideology of retaining or
reconstructing the ligamentous apparatus of the hip joint.
DECLARATIONS
This
work submits to the ethical standards of the Muscles, Ligaments and Tendons
Journal [61]. All data and material are available upon request from the
corresponding author.
CONTRIBUTIONS
S.V.A –
design of investigation, collecting the data and it’s systematization, analysis
the data, writing a draft; D.V.S – conception and design of investigation,
system analysis the data, revising the text; S.V.A & D.V.S – writing text;
all authors discussed the results and commented on the manuscript at all
stages.
CONFLICT
OF INTEREST
The
authors declare that they have no conflict of interest.
REFERENCES
1. Judet
J, Judet R. The use of an artificial femoral head for arthroplasty of the hip
joint. J Bone Joint Surg Br 1950;32(2):166-73.
2.
Bohlman HR. Replacement reconstruction of the hip. Amer J Surg 1952;94(3):268-78.
3.
Thompson FR. Vital hip intramedullary prosthesis - preliminary report. NY State
J Med 1952;52(24):3011-20.
4.
McKeever DC. Biomechanics of hip prostheses. Clin Orthop 1961;19:87-199.
5. McKee
GK, Watson-Farrar J. Replacement of arthritic hips by the McKee-Farrar
prosthesis. J Bone Joint Surg Br 1966;48(2):245-59.
6.
Harmon PH. Joint mobilizing operations on the hip, knee and shoulder for
complications following trauma. Amer J Surg 1947;74(5):598-613.
7. Law
WA. Post-operative Study of Vitallium Mould Arthroplasty of the Hip Joint. J
Bone Joint Surg Br 1948;30B:76-83.
8.
Stinchfield FE, Carroll RE. Vitallium-cup Arthroplasty of the Hip Joint: An
End-Result Study. J Bone Joint Surg Am 1949;31А(3):628-38.
9.
Newman PH, Scales JT. The unsuitability of polythene for movable weight-bearing
prostheses. J Bone Joint Surg Br 1951;33B(3):392-8.
10.
D'Aubigné RM. Reposition with arthroplasty for congenital dislocation of the
hip in adults. J Bone Joint Surg Br 1952;34B(1):22-9.
11.
Nissen KI. The Judet Arthroplasty of the Hip via Gibson's Lateral Approach.
Postgrad Med J 1952.28(321):412-23.
12.
Buxton SJ, Waugh W. Complications and difficulties of the Judet arthroplasty. J
Bone Joint Surg Br 1953;35B(1):57-69.
13.
Thompson FR. Experiences with a vitallium intramedullary hip prosthesis. Tex
State J Med 1953;49(10):749-56.
14.
Shapiro GS, Weiland DE, Markel DC, et al. The use of a constrained acetabular
component for recurrent dislocation. J Arthroplasty 2003;18(3):250-8.
15.
Derar H, Shahinpoor M. Recent patents and designs on hip replacement
prostheses. Open Biomed Eng J 2015;9:92-102.
16.
Binns M. Thompson hemi-arthroplasty through a trochanteric osteotomy approach.
Injury 1985;16(9):595-8.
17.
Fender D, Harper WM, Gregg PJ. Outcome of Charnley total hip replacement across
a single health region in England: the results at five years from a regional
hip register. J Bone Joint Surg Br 1999;81(4):577-81.
18.
Berry DJ, Von Knoch M, Schleck CD, et al. Effect of femoral head diameter and
operative approach on risk of dislocation after primary total hip arthroplasty.
J Bone Joint Surg Am 2005;87(11):2456-63.
19. Meek
RM, Allan DB, McPhillips G, et al. Epidemiology of dislocation after total hip
arthroplasty. Clin Orthop Relat Res 2006;447:9-18.
20.
Patel PD, Potts A, Froimson MI. The dislocating hip arthroplasty: prevention
and treatment. J Arthroplasty 2007;22(4 Suppl 1):86-90.
21.
Parvizi J, Picinic E, Sharkey PF. Revision total hip arthroplasty for
instability: surgical techniques and principles. J Bone Joint Surg Am
2008;90(5):1134-42.
22.
Dargel J, Oppermann J, Brüggemann GP, et al. Luxationen nach Hüftendoprothese.
Dtsch Arztebl Int 2014;111(51-52):884-90.
23.
Hedlundh U, Sanzen L, Fredin H. The prognosis and treatment of dislocated total
hip arthroplasties with a 22 mm head. J Bone Joint Surg Br
1997;79(3):374-8.
24.
Berry DJ, von Knoch M, Schleck CD, et al. The cumulative long-term risk of
dislocation after primary Charnley total hip arthroplasty. J Bone Joint Surg Am
2004;86(1):9-14.
25. Kwon
MS, Kuskowski M, Mulhall KJ, et al. Does surgical approach affect total hip arthroplasty
dislocation rates? Clin Orthop Relat Res. 2006;447:34-8.
26.
Amlie E, Høvik Ø, Reikerås O. Dislocation after total hip arthroplasty with 28
and 32-mm femoral head. J Orthop Traumatol 2010;11(2):111-5.
27. Van
Steenbrugghe H.-G. Nouvelle prothèse articulaire à usage chirurgical [New joint
replacement for surgical use] FR Patent 1047640A, Jan. 10 1952. 1953.
28.
Charnley J. Arthroplasty of the hip. A new operation. Lancet
1961;277(7187):1129-32.
29.
Charnley J. The long-term results of low-friction arthroplasty of the hip
performed as a primary intervention. J Bone Joint Surg Br 1972;54(1):61-76.
30.
Pellet LL. Hip arthroplasty with flexible securing means U.S. Patent 2,765,787
A, August 2, 1954. 1956.
31.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for
systematic reviews and meta-analyses: the PRISMA statement. Int J Surg
2010;8(5):336-41.
32.
Dudko GE. Experience with using polymer-metal endoprosthesis in the treatment
of medial fractures of the femur neck in elderly patients [Опыт применения
полимерно-металлического эндопротеза в лечении медиальных переломов шейки
бедренной кости у пожилых]. Ortop Travmatol Protez 1990;(2):46-8.
33.
Neverov VA, Shil'nikov VA. A method for forming an artificial ligament for the
femur head in endoprosthesis [Способ формирования искусственной связки головки
бедра при эндопротезировании]. Vestn Khir Im I I Grek 1993;151(7-12):81-3.
34.
David T. Prosthetic device for use as a hip joint U.S. Patent 4,092,741 A, June
26, 1975. 1978.
35.
Dudko GE. Method of endoprosthetics of proximal end of femur [Способ
эндопротезирования проксимального конца бедра] S.U. Patent 1,551,366 А1,
October 29, 1986. 1990.
36.
Perepichka VD. Endoprosthesis of proximal epimethaphysis of femur bone
[Эндопротез проксимального эпиметафиза бедренной кости] S.U. Patent 1,572,603
А1, July 6, 1987. 1990.
37.
Neverov VA, Shilnikov VA. Method for plastic surgery on the femoral head in
applying hip joint endoprosthesis [Способ пластики связки головки бедренной
кости при эндопротезировании тазобедренного сустава] S.U. Patent 1,743,595 А1,
May 3, 1990. 1992.
38.
McCandliss R. Coaxial ligamented hip prosthesis. U.S. Patent 5,702,474 A,
January 22, 1996. 1997.
39.
Dennis DA, Komistek RD. Method and apparatus for hip prosthesis. U.S. Patent
5,951,605 A, July 1, 1996. 1999.
40. Shah
MK. Joint replacement system U.S. Patent 6,010,535 A, April 30, 1998. 2000.
41.
Pedersen WB, Steenstrup FR, Olsen OI, et al. A prosthetic device U.S. Patent
7,993,566 B2, December 17, 1999. 2011.
42.
Stinnette A. Socket and prosthesis for joint replacement U.S. Patent 7,909,882
B2, January 19, 2007. 2008.
43.
Lozier AL, Parrott RM, Rich DB. Joint space interpositional prosthetic device
with internal bearing surfaces U.S. Patent 8,979,935 B2, July 31 2007. 2010.
44.
Linares MA. Hip socket with assembleable male ball shape having integrally
formed ligament and female receiver and installation kit U.S. Patent 8,211,182
B2, September 17, 2007. 2010.
45.
Linares MA. Artificial ligaments for joint applications U.S. Patent 7,887,586
B2, September 17, 2007. 2011.
46.
Frederick P, Belew K, Jasper L, et al. Methods and apparatus for FAI surgeries
U.S. Patent 8,900,320 B2, February 24, 2009. 2014.
47.
Forsell P. Hip joint device and method U.S. Patent 9,138,320 B2, July 10, 2009.
2015.
48.
Komistek RD. Maintaining proper mechanics THA U.S. Patent 9,023,112 B2,
February 24, 2011. 2015.
49.
Castro FF, Fisher JMO, Moskovitz AP. Semi-constrained ball and socket joints
U.S. Patent 9,060,862 B2, July 8, 2011. 2013.
50.
Birmingham P. Method and device for joint replacement U.S. Patent 10,064,730
B2, March 13, 2015. 2018.
51.
Haining Z. Artificial total hip joint prosthesis with axially restrained
anti-dislocation structure. CN. Patent 105,105,873 B, August 7, 2015. 2015.
52.
Boroumand S, Halwai I. Tanab-Ligament [Eine bandartige Struktur, die die
Luxation einer Gelenkprothese verhindert]. DE Utility model 20,2015,006,363 U1,
September 9, 2015. 2015.
53.
Urist MR. Hip socket means. U.S. Patent 2,910,978 A, March 28, 1955. 1959.
54.
Botha PJ. Joint prosthesis component WO 2006/030392 A1, September 17, 2004.
2006.
55.
Nicholl JE, Bintcliffe IWL. Recurrent dislocation of a hemiarthroplasty
stabilized with an ABC ligament. Injury 1996;27(6):447-8.
56.
Fujishiro T, Nishikawa T, Takikawa S, et al. Reconstruction of the iliofemoral
ligament with an artificial ligament for recurrent anterior dislocation of
total hip arthroplasty. J Arthroplasty 2003;18(4):524-7.
57.
Barbosa JK, Khan AM, Andrew JG. Treatment of recurrent dislocation of total hip
arthroplasty using a ligament prosthesis. J Arthroplasty 2004;19(3):318-21.
58.
Allington NJ, Ronda J. Use of synthetic ligament in reconstruction after
massive bone tumour removal. Acta Orthop Belg 2012;78(2):263-6.
59.
Hardes J, Ahrens H, Nottrott M, et al. Der Anbindungsschlauch zur
Weichteilrekonstruktion nach Megaprothesenimplantation [Attachment tube for
soft tissue reconstruction after implantation of a mega-endoprosthesis]. Oper
Orthop Traumatol 2012;24(3), 227-34.
60. Du
Z, Tang S, Yang R, et al. Use of an artificial ligament decreases hip
dislocation and improves limb function after total femoral prosthetic
replacement following femoral tumor resection. J Arthroplasty
2018;33(5):1507-14.
61. Padulo
J, Oliva F, Frizziero A, et al. Muscles, Ligaments and Tendons Journal – Basic
principles and recommendations in clinical and field Science Research: 2018
update. MLTJ 2018;8(3):305–307.
Authors
& Affiliations
Authors:
S.V.
Arkhipov (1, 2)
D.V.
Skvortsov (1)
Organization:
1)
Laboratory of Clinical Physiology and Biomechanics, Federal State Budgetary
Institution “N.N. Priorov National Medical Research Center of Traumatology and
Orthopaedics” of the Ministry of Health of the Russian Federation, Moscow,
Russia1.
2) MEDSI
Group of Companies, Surgical Department, Solyanka str. 12/1, 109240, Moscow,
Russia
External
links
Arkhipov SV, Skvortsov DV. Ligamentum Teres and its Analog in the Hip Endoprosthesis–Necessary or Superfluous? A Systematic Review. MLTJ. 2021:11(2)301-10. doi:10.32098/mltj.02.2021.13 [researchgate.net(PDF) , mltj.online]

.jpg)
Comments
Post a Comment